The
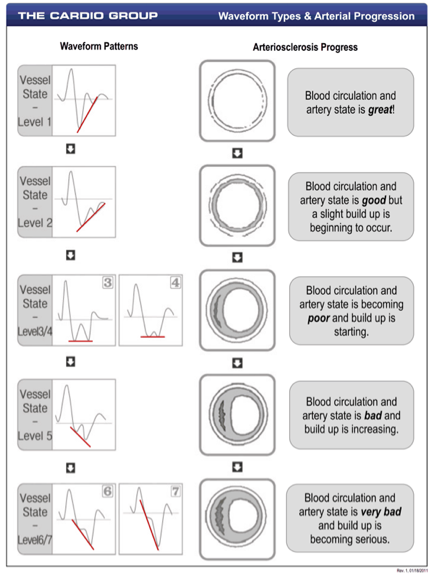
Finder Test To Measure The Elasticity Of Arterial System
The
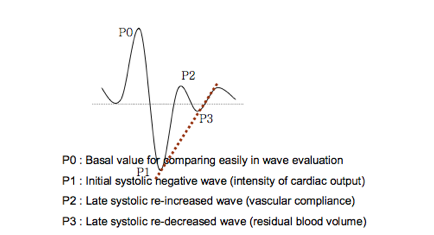
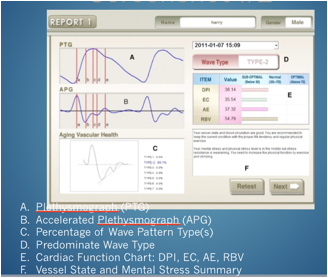
accelerated photoplethsmograph shines light through the vascular bed of the
finger measuring the thrust of the heart beat wave of blood and the rebound of
blood off the vascular walls. The
test measures each heart beat during a 3 minute period so that about 200 heart
beats are analyzed. These beats
wave forms are evaluated and a summation graph is depicted on a screen for
evaluation.
The
test is quick, painless and takes a surprisingly good estimate of the vascular
health of a person.
The
lining of the vascular tree is a one cell deep and lines every blood vessel and
capillary in your body from your intestines, to your brain, to your legs and
heart. Your endothelium is a
significant part of your body.
The
surface endothelial cells communicate with
smooth muscles below that open and close your arteries. The communication is with a naturally
occurring gas called nitric oxide, NO.
The
gas NO tells your blood vessels to relax, to open up, and let the blood flow
and reduce blood pressure.
Dr.
Louis Ignarro is a professor at UCLA department of Medicine and
Pharmacology. He discovered that
the endothelial cells and other cells of the body of all animals use this gas
to regulate their vascular system.
In 1997 received the Nobel Prize in Medicine for his 30 years of
investigative work.
What
Dr. Ignarro learned is that diseased blood vessels have sick endothelium that
does not make enough NO.
If
you give the blood vessels the right diet and exercise, the vessels can stop
getting sicker and can repair.
Dr.
Ignarro discovered that if you give molecules that cause the body to make more
NO, the blood vessels heal. They
heal relatively quickly, and signs of vascular disease such as elevated blood
pressure is reduced and the elasticity can return to the stiffened blood
vessels. It is a miraculous
discovery.
The
precursor for NO is an amino acid named arginine. Amino acids make up proteins. When you eat a steak or turkey or
chicken meat, 5 per cent of the protein is made up of the amino acid arginine.
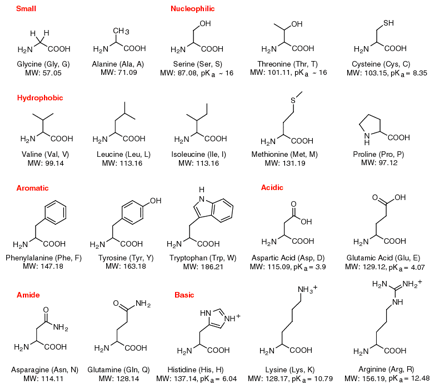
The
above table shows the 20 amino acids that make up most protein. Can you find arginine?
Dr.
Ignarro discovered that high concentrations of arginine along with another
amino acid, citruline, produce accelerated healing of blood vessels. Changes are apparent after 90 days on
the photplethsmograph. But more
than that, blood pressure is down in people with high blood pressure, many
times only after two weeks after taking arginine with Dr. IgnarroÕs suggestion
and people feel better.
Because
arginine is an amino acid, which essentially is a basic building block of our
food, its structure cannot be patented. There can be no monopoly, no
matter how much the substance can help people reverse blood vessel disease or atherosclerosis
and heal their vessels. Large drug
companies have no interest in selling it or marketing it.
Dr.
Ignarro in his book NO More Heart Attacks And Strokes printed the
optimal recipe for how much citruline and how much arginine a person should
take for maximum response as a result of his research. And Dr. Ignarro went to Herbal Life, a
large manufacturer and marketer of vitamins with his formulations. He went with a businessman and a product
at Herbal Life was produced with Dr. IgnarroÕs recommendations and his
endorsements. Other vitamin
companies copied and expanded on his basic idea. He published in his book what he had learned from his research is
the ideal mix of arginine and cituline for optimum repair of vessels. The cat was out of the bag. Any vitamin maker could copy his
mixture. One such company is Synergy with their ProArgi-9+ formulation that has added
vitamin D, B12, and other B vitamins.
The
magic dose of arginine is 3 to 4 grams twice a day and of citruline, 1 to 2
grams twice per day. Dr. Ignarro
directly states in his book that any other combination or arginine alone is a
waste of money with no benefit.
Basically
this paper describes two miraculous finding. A photplethsmograph can evaluate your
endothelial arterial health so quick and easily you do not at first understand
how important is the information you have.
And
a solution to vascular disease; A simple food supplement that actually works. 7 out of ten people will die of
complications from vascular disease such as a heart attack or a stroke. Only 3 out of ten will die from cancer.
Dr.
Ignarro



If you want a simple explanation of how you can
reverse athersclerosis you should buy Dr. IgnarroÕs book for $12 on Amizon.

You should definitely read this book. It was written with love to keep all 2
billion of you healthy.
Louis J. Ignarro (born May 31, 1941) is an American pharmacologist. For demonstrating the signaling properties
of nitric oxide, he was co-recipient of the
1998 Nobel Prize in Physiology or
Medicine
with Robert F. Furchgott and Ferid Murad.
Currently, he is professor
of pharmacology at the UCLA School of Medicine's department of molecular
and medical pharmacology in Los Angeles, which he joined in 1985. Before
relocating to California, he was a professor of pharmacology at Tulane University School of Medicine, New
Orleans, for 12 years. Ignarro has also previously worked as a staff scientist,
research department, for the pharmaceutical division of CIBA-GEIGY Corporation in New York.
Ignarro has published
numerous research articles. He received the Basic Research Prize of the American Heart Association in 1998. This was in
recognition of his outstanding contributions to the advancement of
cardiovascular science. That same year, he was inducted into the National
Academy of Sciences and the following year, into the American Academy of Arts
and Sciences. Because nitric oxide is indirectly involved in the action of this
drug, he is sometimes referred to as the "Father of Viagra".[1]
He is the founder of the
Nitric Oxide Society, and founder and editor-in-chief of Nitric Oxide Biology and Chemistry.[2] Ignarro holds a B.S. in
pharmacy, Columbia University, 1962, and a Ph.D. in
pharmacology, University of Minnesota, School of Medicine, 1966.
He also received a postdoctoral fellowship in chemical pharmacology from National Institutes of
Health in
1968. He is a member of the scientific committee of Nicox, a French pharmaceutical company, a member of the
Board of Directors of Antibe Therapeutics,[3] a Canadian drug discovery
company, a member of the Board of Directors of Operation USA, a non-profit organization, and a member of the
Nutritional Advisory Board for Herbalife, a for-profit nutrition and weight-management company
Personal life
Louis J. Ignarro was born in
1941 in Brooklyn, New York. His parents were Italian
immigrants and his father was a carpenter in Torre del Greco, near Naples. Ignarro grew up in Long Beach, NY, which is a suburb of New York City, NY on the south shore of Long Island, NY. Ignarro received his first
chemistry set as a gift at the age of 8.[4]
Ignarro is married and lives
in Beverly Hills, CA.[4] He is an avid cyclist and
marathoner, having completed 13 marathons.[5][6] Ignarro has published
multiple books for lay audiences about health and wellness focusing on the
benefits of increasing nitric oxide production. He is a frequent public speaker
on these and related topics.
Academic career
Ignarro attended Central
Grade School and Long Beach High School. A strong interest in science led
Ignarro to Columbia University where he studied chemistry
and pharmacology and in 1962 received a bachelor's degree in pharmacy. Ignarro
then attended the University of Minnesota where he received a Ph.D.
in pharmacology. His university studies also concentrated in chemistry,
enzymology and cardiovascular physiology, which resulted in several published
papers. While at the University of Minnesota, Ignarro studied under eventual
Nobel Prize winning chemist Paul Boyer.[4]
Ignarro's work continued at
the NIH in the fields he'd studied, collaborating with many
other scientists to discover regulatory mechanisms of the cardiovascular system
that would lead to his most famous work. This was his first time to apply his
education outside of an academic setting. In 1968, Ignarro left the NIH to work
for Geigy Pharmaceuticals. With this company, Ignarro helped develop new drugs
and was able to continue research into new areas of pharmacology including
cyclic GMP. After Geigy merged with Ciba Pharmaceuticals, Ignarro decided to
move back to the world of academia, this time as a professor.[4]
In 1973, Ignarro accepted
the position of Assistant Professor of pharmacology at Tulane University School of Medicine in New Orleans, LA. Tulane was chosen
partially because it would provide a good environment for continued research into
cyclic GMP. While studying cyclic GMP, Ignarro read a paper by Ferid Murad, who demonstrated that nitric oxide elevates cyclic
GMP levels. Ignarro then speculated that nitric oxide could be the key to
relaxing vascular smooth muscles. In turn, this led to his extensive research
on the subject. Ignarro's research demonstrated that nitric oxide serves the
functions of vasorelaxant and inhibitor of platelet aggregation, with both
effects mediated by cyclic GMP.
Ignarro continued his
research at Tulane. In 1984 he realized that the properties of nitric oxide
were the same as those seen in the endothelium derived relaxing factor
previously-identified by Robert Furchgott 3 years earlier. The exact nature of
the EDRF was up to this point unknown. Interestingly, Furchgott and Ignarro
came to similar conclusions about nitric oxide as the EDRF around the same time
and both presented evidence at conferences during 1986 demonstrating nitric
oxide's role as EDRF.[4]
During the decades since
Ignarro and Furchgott's initial research, thousands of studies have been
published about the effects of nitric oxide as the endothelium derived relaxing
factor. This has led to the development of erectile dysfunction drugs such as Viagra and nutritional supplements designed for
cardiovascular health and athletic performance. In 1985, Ignarro moved from New
Orleans to Los Angeles where he accepted a position at the UCLA School of Medicine and continues to research and
teach.[4]
Herbalife relationship
Ignarro has worked as a paid
consultant for Herbalife since 2003 and later became
a member of the company's Scientific Advisory Board. He has collaborated in
developing nutritional supplements for cardiovascular health and athletic
performance. Ignarro first worked with Herbalife to develop Niteworks, a dietary supplement designed
to boost the bodyÕs own production of nitric oxide.[7] Ignarro endorsed this
product in exchange for a royalty agreement reported to have earned his
consulting firm over $1 million in the first 12 months.[8] Ignarro has continued to
work with Herbalife to develop additional supplements focusing on nutrients
such as Omega-3 fatty acid and CoQ10. As of 2012, Herbalife has made payments to Ignarro
and his affiliated consulting firm of over $15 million.[9]
In 2003, Ignarro published a
study based on ingredients found in Niteworks in the Proceedings of the National
Academy of Sciences, where, as a member of the National Academy of Sciences, he can submit papers
without review and without disclosing his financial interest to the
publication. After Ignarro's ties to Herbalife were revealed, the journal
issued a correction to the article, citing Ignarro's undisclosed "conflict
of interest." [8] UCLA conducted its own
investigation and determined that Ignarro did not act improperly. Ignarro has
publicly acknowledged the conflict of interest as a mistake and has continued
to published extensively on the subject of nitric oxide.[10]
Ignarro appears in videos
promoting Niteworks and other Herbalife products, and is a frequent speaker at
Herbalife events. Since partnering with Herbalife, Ignarro has spoken to more
than 5,000,000 people worldwide about nitric oxide and cardiovascular health.[10]
Famous quotes
While testifying before
Congress in 2000, Ignarro remarked: "Only in America could the son of an
uneducated carpenter receive the Nobel Prize in Medicine".[11][12]
Awards and recognitions
- Pharmaceutical
Manufacturers Association Foundation Research Award. 1973
- Merck
Research Award. 1974 [13]
- Edward
G Schlieder Foundation Award. 1973–1976 [13]
- U.S.P.H.S.
Career Development Award. 1975 – 1980
- Arthritis
Foundation Research Award. 1975 – 1977
- First
recipient of the George Clark Memorial Arthritis Fund. 1975
- First
recipient of the James Woodrow Waggoner Arthritis Fund. 1976
- Lilly
Research Award. 1978 [13]
- Tulane
Medical School – Outstanding Teacher Award. 1983
- UCLA
School of Medicine – Outstanding Teacher Award. 1986
- AMSA
Golden Apple Award (for teaching) – UCLA School of Medicine. 1987,
1988, 1989, 1990, 1991, 1992, 1993, 1994, 1995, 1996, 1997, 1999
- Alpha Omega Alpha – Honorary
Member. 1990
- UCLA
School of Medicine Award for Excellence in Education. 1993
- Roussel
Uclaf Prize for Cell Communication and Signaling. Shared with Dr. Salvador
Moncada and Dr. Robert Furchgott. 1994 [2]
- Wellcome
Visiting Professor – Marshall University School of Medicine. 1995
- CIBA
Award for Hypertension Research for Discovery of the Roles of Nitric Oxide
and Cyclic GMP in Vascular Function. Shared with Dr. Salvador Moncada.
1995[2]
- Basic
Research Prize of the American Heart Association in recognition of
outstanding contributions to the advancement of cardiovascular science.
1998
- Nobel
Prize in Physiology or Medicine for Ònitric oxide as a signaling molecule
in the cardiovascular systemÓ. Shared with Robert Furchgott and Ferid
Murad. 1998
- Laurea
honoris causa(Honorary degree), University of Naples
Federico II. 1999[14]
- National Academy of
Sciences.
1999
- American Academy of
Arts and Sciences. 1999
- Institute of Medicine. 2011
- American
Philosophical Society. 2007
- Canadian
Medal of Merit. 2008[15]
- American
Heart Association Distinguished Scientist. 2008[16]
- Honorary
doctorates from the Universities of Madrid, Lund, Gent, North Carolina [13]
Primary Required viewing.
Dr. Louis Ignarro discusses arginine and NO part one
https://www.youtube.com/watch?v=DcIWX8C91s4
Dr.
Louis Ignarro discusses arginine part 2
https://www.youtube.com/watch?feature=endscreen&NR=1&v=NBPjZJSHr4A
How Dr. Louis Ignarro changed his own life because of his studies.
https://www.youtube.com/watch?v=MH03jAT5KNY&feature=endscreen&NR=1
Dr.
Louis Ignarro on effects of NO, and how and when is NO made in the human body.
https://www.youtube.com/watch?v=8w1e5saAXTg
argenene
Sources[edit]
Dietary sources[edit]
Arginine is a conditionally nonessential amino acid,
meaning most of the time it can be manufactured by the human body, and does not
need to be obtained directly through the diet. The biosynthetic pathway however
does not produce sufficient arginine, and some must still be consumed through
diet. Individuals who have poor nutrition or certain physical conditions may be
advised to increase their intake of foods containing arginine. Arginine is
found in a wide variety of foods, including:[5]
¥
Animal sources
dairy products (e.g., cottage cheese, ricotta, milk, yogurt, whey protein drinks), beef, pork (e.g., bacon, ham), gelatin , poultry (e.g. chicken and turkey light meat), wild game (e.g. pheasant, quail), seafood (e.g., halibut, lobster, salmon, shrimp,
snails, tuna)
¥
Plant sources
wheat germ and flour,
buckwheat, granola, oatmeal, peanuts, nuts (coconut, pecans, cashews, walnuts, almonds,
Brazil nuts, hazelnuts, pinenuts), seeds (pumpkin, sesame, sunflower), chick peas, cooked soybeans, Phalaris canariensis
(canaryseed or ALPISTE)
Biosynthesis[edit]
Arginine is
synthesized from citrulline by the sequential
action of the cytosolic enzymes argininosuccinate synthetase
(ASS) and argininosuccinate lyase
(ASL). In terms of energy, this is costly, as the synthesis of each molecule of
argininosuccinate requires hydrolysis of adenosine triphosphate
(ATP) to adenosine monophosphate
(AMP), i.e., two ATP equivalents. Taking an excess of arginine essentially
gives more energy by saving ATPs that can be used elsewhere.
Citrulline can be
derived from multiple sources:
¥
from arginine via nitric oxide synthase (NOS)
¥
from ornithine via catabolism of proline or glutamine/glutamate
¥
from asymmetric dimethylarginine
(ADMA) via DDAH
The pathways
linking arginine, glutamine, and proline are bidirectional. Thus, the net utilization
or production of these amino acids is highly dependent on cell type and
developmental stage.
On a whole-body
basis, synthesis of arginine occurs principally via the intestinal–renal
axis, wherein epithelial cells of the small
intestine, which produce citrulline primarily from glutamine and glutamate, collaborate with the proximal tubule cells of the kidney, which extract citrulline from the circulation
and convert it to arginine, which is returned to the circulation. As a
consequence, impairment of small bowel or renal function can reduce endogenous
arginine synthesis, thereby increasing the dietary requirement.
Synthesis of
arginine from citrulline also occurs at a low level in many other cells, and
cellular capacity for arginine synthesis can be markedly increased under
circumstances that also induce iNOS. Thus, citrulline, a
coproduct of the NOS-catalyzed reaction, can be recycled to arginine in a
pathway known as the citrulline-NO or arginine-citrulline pathway. This is
demonstrated by the fact that in many cell types, citrulline can substitute for
arginine to some degree in supporting NO synthesis. However, recycling is not
quantitative because citrulline accumulates along with nitrate and nitrite, the
stable end-products of NO, in NO-producing cells.[6]
Function[edit]
Arginine plays an
important role in cell division, the healing of wounds, removing ammonia from
the body, immune function, and the release of hormones.[3][7][8]
The benefits and
functions attributed to oral supplementation of L-arginine include:
¥
Precursor for the
synthesis of nitric oxide (NO)[9]
¥
Reduces healing
time of injuries (particularly bone)[7][8]
¥
Quickens repair
time of damaged tissue[7][8]
¥
Helps decrease blood pressure in clinical hypertensive subjects [10][11][12]
Proteins[edit]
The distributing
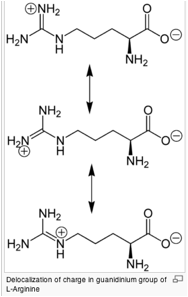
basics of the moderate structure found in geometry, charge distribution and
ability to form multiple H-bonds make arginine ideal for binding negatively
charged groups. For this reason, arginine prefers to be on the outside of the
proteins where it can interact with the polar environment.
Incorporated in
proteins, arginine can also be converted to citrulline by PAD enzymes. In
addition, arginine can be methylated by protein methyltransferases.
Precursor[edit]
Arginine is the
immediate precursor of nitric oxide (NO), urea, ornithine, and agmatine; is necessary for the synthesis of creatine; and can also be used for the synthesis of polyamines (mainly through ornithine and to a lesser
degree through agmatine), citrulline, and glutamate. As a precursor of nitric oxide, arginine may
have a role in the treatment of some conditions where vasodilation is required.[3] The presence of asymmetric dimethylarginine
(ADMA), a close relative, inhibits the nitric oxide reaction; therefore, ADMA
is considered a marker for vascular
disease, just as L-arginine
is considered a sign of a healthy endothelium.
Treatment of dentin hypersensitivity[edit]
Arginine (8%) in
dental products (e.g., toothpaste) provides
effective relief from sensitive teeth by depositing a dentin-like mineral, containing calcium and phosphate, within the dentin tubules and in a protective
layer on the dentin surface.[13]
Treatment of herpes simplex virus[edit]
An unproven claim
is that a low ratio of arginine to lysine may be of benefit in the treatment of herpes
simplex virus. For more information, refer to Herpes -
Treatment also see journal
article.[14]
Possible increased
risk of death after supplementation following heart attack[edit]
A clinical trial
found that patients taking an L-arginine supplement following a heart attack
found no change in the heart's vascular tone or decrease in the symptoms of congestive heart failure
(the heart's ability to pump). In fact, six more patients who were taking L-arginine
died than those taking a placebo resulting in early termination of the study
with the recommendation that the supplement not be used by heart attack
patients.[15][16][17] These findings suggest L-arginine
is not beneficial post-heart-attack.
Potential medical
uses[edit]
Lung inflammation and asthma[edit]
Inhalation of L-arginine
can increase lung inflammation and worsen asthma.[18]
Growth hormone[edit]
Intravenously-administered
arginine stimulates the secretion of growth hormone,[19] and is used in growth
hormone stimulation tests.[20] Research suggests that oral preparations of L-arginine
are ineffective at increasing growth hormone levels despite being effective at
increasing plasma levels of L-arginine.[21]
MELAS syndrome[edit]
Several trials
delved into effects of L-arginine in MELAS syndrome, a mitochondrial disease.[22][23][24][25]
Sepsis[edit]
Cellular arginine
biosynthetic capacity determined by activity of argininosuccinate synthetase
(AS) is induced by the same mediators of septic response — endotoxin and cytokines — that induce nitric oxide synthase (NOS), the enzyme responsible for nitric oxide synthesis.[26]
Malate salt[edit]
The malate salt of arginine can also be used during the
treatment of alcoholic hepatitis and advanced cirrhosis.[27]
Pre-eclampsia[edit]
A preliminary
study of supplementation with L-arginine and antioxidant vitamins showed that
this combination may help to combat abnormally high blood pressure during high
risk pregnancies.[28]
Hypertension[edit]
Intravenous
infusion of arginine reduces blood pressure in patients with hypertension as
well as normal subjects.[29]
A recent
meta-analysis showed that L-arginine reduces blood pressure with pooled
estimates of 5.4/2.7 mmHg for SBP/DBP.[12]
Erectile
dysfunction[edit]
Arginine taken in
combination with proanthocyanidins[30] or yohimbine,[31] has also been
used as a treatment for erectile dysfunction.
Anxiety[edit]
Dietary supplementation
of L-arginine taken in combination with L-lysine has been shown potentially useful in treating
people subjected to high levels of mental stress and anxiety, in a double-blind, placebo controlled and
randomized study, involving 108 Japanese adults. Trait anxiety and state
anxiety induced by cognitive stress battery was significantly reduced, and
basal levels of
Dr.
Ignarro discusses how the body makes NO
https://www.youtube.com/watch?v=Gmcj88ysk1A
Dr.
Ignarro discusses which came first disease or deficience of NO?
https://www.youtube.com/watch?v=szCnaCK1Zv8
Dr.
Ignarro speaks again, on NO made by
the body itself with exercise, as well suppliments and vitamins
https://www.youtube.com/watch?v=h8Q_tFU8YtM
citruline
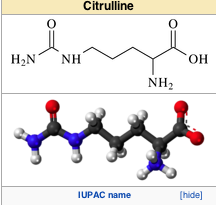
Biosynthesis [edit]
Citrulline is made
from ornithine and carbamoyl
phosphate in one of the central
reactions in the urea cycle. It is also produced from arginine as a by-product of the reaction catalyzed by NOS family (NOS; EC
1.14.13.39).[3] It is made from arginine by the enzyme trichohyalin at the inner root sheath and medulla of hair
follicles.[4] Arginine is first oxidized into
N-hydroxyl-arginine, which is then further oxidized to citrulline concomitant
with release of nitric oxide.
Function [edit]
Although human DNA does not code for citrulline directly, several
proteins contain citrulline as a result of a posttranslational modification.
These citrulline residues are generated by a family of enzymes called
peptidylarginine deiminases (PADs), which convert arginine into citrulline in a
process called citrullination or deimination.
Proteins that normally contain citrulline residues include myelin basic protein (MBP), filaggrin, and several histone proteins, whereas other proteins, such as fibrin and vimentin are susceptible to citrullination during cell
death and tissue inflammation.
Patients with rheumatoid arthritis often have
detectable antibodies against proteins containing citrulline. Although the
origin of this immune response is not known, detection of antibodies reactive
with citrulline (anti-citrullinated protein antibodies) containing proteins or peptides is now becoming an important help
in the diagnosis of rheumatoid arthritis.[5]
In recent studies,
citrulline has been found to relax blood vessels.[6] Circulating
citrulline concentration is, in humans, a biomarker of intestinal
functionality.[7]
Sources [edit]
Citrulline in the
form of citrulline malate is sold as a
performance-enhancing athletic dietary
supplement, which was shown to
reduce muscle fatigue in a preliminary clinical trial.[8]
The rind of watermelon (Citrullus lanatus) is a good natural
source of citrulline.[9]
Dr. Ignarro discusses his Nobel prize ceremony and how Argenine reverses
heart disease
https://www.youtube.com/watch?v=EcvP_MTJ4Wo
Proarg9+
high desert heart study
Dr.
Silva Arunasalam is a cardiologist who treats sick people with heart
disease. He is the director of the
high desert Heart Institute in Hemet California, a group of 5 cardiologists.
This group of physicians headed by Dr.
Silva, as he likes to called, had 33 patients with severe heart failure. Their hearts were so weak each patient
was put on the heart transplant list.
They were on maximal medical treatment.
Dr. Siva treated all 33 patients with a
regimen that followed Dr. IgnarroÕs formulation. And over a course of a year all the
patients improved with the supplement well enough to be able to be taken off
the heart transplant list.
https://www.youtube.com/watch?v=Avpco86uStI


Siva
Arunasalam, M.D.
Personal History
|
Business
address: |
|
Heart
Institute of the High Desert 12332 Hesperia
Road Victorville,
California 92392 email
– puravi@aol.com |
|
Business
telephone: |
|
(760)
241-2270 |
|
Business
FAX: |
|
(760)
241-4081 |
|
|
|
|
|
Education |
|
|
|
1977 to
1980 |
|
University
of Nebraska, Lincoln, B.S., Magna cum
laude |
|
1980 to
1981 |
|
University
of Nebraska, Lincoln, M.S. |
|
1982 to
1987 |
|
Emory
University School of Medicine, M.D. |
|
1987 to
1988 |
|
Internship, Harbor-UCLA Medical Center, Torrance, CA |
|
1988 to
1990 |
|
Residency, Harbor-UCLA Medical Center, Torrance, CA |
|
1990 to
1991 |
|
Fellowship, Cedars‑Sinai Medical Center, Los Angeles, CA, Kenemer
Fellowship in Critical Care Medicine, Emphasis – drug use and
laboratory studies |
|
1991 to
1994 |
|
Fellowship, Cardiology, Cedars-Sinai Medical Center, Los Angeles, CA |
|
|
|
|
|
Licensure: |
|
California
– G066022 |
|
Board
Certification: |
|
ABIM,
Internal Medicine, 1990-2000 (135007) Recertified,
2000-2010 (135007) |
|
|
|
ABIM,
Cardiovascular Disease, 1995-2004 (135007) Recertified,
2004-2014 |
|
|
|
|
|
DEA Number: |
|
BA1579937 |
|
Medicare
Number: |
|
00G660220 |
|
MediCal
Number: |
|
00G538411 |
|
UPIN |
|
E32683 |
|
Fluroscopy
and Radiography |
|
RHD 135056 |
|
|
|
|
|
Title: |
|
President
and Attending Cardiologist, High Desert
Heart Institute Victorville,
California |
Professional Experience
|
September
1996 to present |
|
President
and Attending Cardiologist Heart
Institute of the High Desert Victorville,
California |
|
July, 1992
to December, 1996 |
|
Attending
Cardiologist Lancaster
Cardiology Medical Group 43847 North
Heaton Avenue, Lancaster, CA 93534 |
|
May, 1993
to July, 1995 |
|
Attending
Cardiologist Cardiology
Associates 8641
Wilshire Blvd, Suite 300 Los
Angeles, CA 90210 Physician
and Lab Director for Moderate Complexity Testing Laboratory |
|
July 1990
to May, 1995 |
|
Cedars-Sinai
Medical Center 8700
Beverly Blvd Los
Angeles, CA 90048 |
|
July, 1996
to June 1999 |
|
Associate
Clinical Professor Step I, Department of Medicine, University of California,
Los Angeles |
|
July, 1990
to July, 1996 |
|
Assistant
Clinical Professor, Department of Medicine, University of California, Los
Angeles |
Professional Activities
|
Societies: |
|
American
Heart Association |
|
|
|
Alpha Omega
Alpha |
|
|
|
San
Bernardino Medical Society |
|
|
|
|
|
Committees: |
|
Chairman,
Critical Care Committee Barstow
Community Hospital – ~1999-2001 |
|
|
|
Vice-Chairman,
Department of Internal Medicine St. Mary
Regional Medical Center Apple
Valley, CA ~1999 |
Honors and Special Awards
|
Magna Cum Laude, University of Nebraska,
Lincoln, 1980 |
|
Phi Beta Kappa, University of Nebraska,
Lincoln, 1980 |
|
Alpha Omega Alpha, Emory University School of
Medicine, 1987. |
Media Appearances
|
Heart
patients get InSync for new life, Daily Press, Victorville, CA, April 3, 2002 |
|
High desert
has a heart for Fiji teen, Daily Press, Victorville, CA, May 15, 2001 |
|
Waiting for
life, Desert Dispatch, Barstow, CA, March 16, 2001 |
Hospital Privileges
|
October
1995 to present |
|
Barstow
Community Hospital 555
South Seventh Street |
|
October
1995 to present |
|
Desert
Valley Hospital 16850
Bear Valley Road Victorville,
CA 92392 |
|
September
1995 to March 2005 |
|
St
Mary Regional Medical Center 18300
Highway 18 Apple
Valley, CA 92307 |
|
September
1995 to present |
|
Victor
Valley Community Hospital 15248
Eleventh Street Victorville,
CA 92392 |
|
July
1990 to June 1995 |
|
Cedars-Sinai
Medical Center 8700
Beverly Boulevard Los
Angeles, CA 90048 |
Publications/Bibliography
1.
Luo
H. Steffen W. Cercek B. Arunasalam S. Maurer G. Siegel RJ. Enhancement of thrombolysis by external ultrasound.
American Heart Journal. 125(6):1564-9, 1993 Jun
2.
Arunasalam S. Siegel RJ. Rapid resolution of symptomatic acute pericarditis with
ketorolac tromethamine: a parenteral nonsteroidal antiinflammatory agent.
American Heart Journal. 125(5 Pt 1):1455-8, 1993 May
3.
Arunasalam S. The evaluation of aortic
dissection and aortic atheromatosis, in Transesophageal Echocardiography,
McGraw-Hill Professional, Chapter 11, January, 1994.
Dr. Arunasalam discusses results with his patients with Proargi9+
http://www.youtube.com/watch?v=DhhCi2C66Rc&feature=youtu.be&goback=.gde_137485_member_211638401
This is a commercial for Proargi9+
This product has extra 2500 units vitamin D per scoop. 4 scoops per day equals 8000 units of
vitamine D
http://www.youtube.com/watch?v=logzbsiW6zs
82
![]()

. Joseph Prendergast has been a practicing physician for over 30 years.
He is Board Certified in Internal Medicine as well as Endocrinology and Metabolism.
A graduate of Wayne State University in Detroit, Michigan he completed a
fellowship in Endocrinology and Metabolism at Henry Ford Hospital Detroit, MI
and his residency at the University of California, San Francisco. Dr.
Prendergast has published over 40 medical articles in well-known publications
such as the Journal of the American Medical Association, The New England
Journal of Medicine and Diabetes Care. In 1986, Dr. Prendergast formed a single
specialty endocrinology practice, (Endocrine Metabolic Medical Center) and a
non-profit research foundation (Pacific Medical Research Foundation). In 1999,
he founded DiabetesWell, an eClinic that managed patients with diabetes to lead
healthier, longer lives.
"Patient empowerment is probably the most philosophically exciting idea to
emerge in medicine in recent years. Patients can, and must, be educated to play
the primary role in maintaining their own health. I have seen it work and have
had the great satisfaction over the past 30 years of helping thousands of
individuals live full and active lives."
- J. Joseph Prendergast, MD.
Specialties:Internal Medicine, Endocrinology, Diabetes.
Dr. Prendergast is a doctor who is an endocrinologist. Endocrinologists see lots and lots of
diabetic patients. Diabetic
patients have lots of vascular complications attributed to their diabetes. Mostly large blood vessel disease
involving the entire body and small blood vessels affecting the brain and eyes
and kidneys. Dr. Prendergast
relates that in his experience with his patients the vascular complication of
diabetes are arrested in patients taking arginine.
Dr. Pendergast discusses his personal history with
arginine therapy.
Dr. Pendergast explains how the arginine citrulline
products works for restoration of sick blood vessels.
http://www.youtube.com/watch?v=a_aPxSlHLF0
Dr. Pendergast repeats his
experience with arginine and his
own family history of vascular disease.
http://www.youtube.com/watch?v=Qq_bkiTVly0
on use of arginine use of arginine after a heart attack in patients
with diabetes.
http://www.youtube.com/watch?v=IFgaZpQtzUk
Arginine products available on Amazon
Dr. Ignarro discusses the
Herbal life product
https://www.youtube.com/watch?v=ZnoegiE17Z8
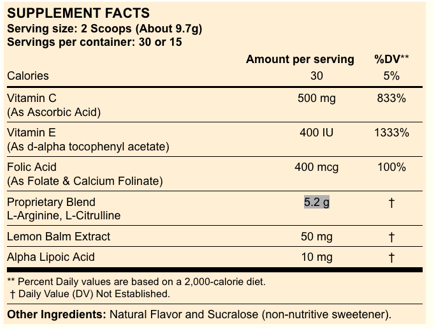
Containes 500 mg vitamine C
400 mcg folic acid
vitamine E 200 IU
L-taurine 300 mg
alfa lipoic acid 10 mg
Do you see Dr. IgnarroÕs signature?

Details
Developed with Nobel Laureate in Medicine Dr. Lou Ignarro, Herbalife
Niteworksš helps your body produce Nitric Oxide (NO), for improved circulatory,
immune and nervous system functions.* L-arginine and l-citrulline trigger
cells to produce and recycle more NO.
Usage
Two scoops (two teaspoons or about 10 grams) in 8 oz. of cold water.
May be mixed with sparkling water or juice. Drink at night or before bedtime.*





1
Dr. Ferid Murad

Ferid Murad (born September 14, 1936) is an Albanian-American physician and pharmacologist, and a co-winner of the 1998 Nobel Prize in Physiology or
Medicine.
He is also an honorary member of the Academy of Sciences and Arts
of Kosovo.[1]
Contents |
Life
He was born in Whiting, Indiana to Jabir Murat Ejupi, an
Albanian immigrant from Gostivar, Macedonia, and Henrietta Bowman, an
American Christian, Ferid Murad was raised as
a Christian.[2] He received his
undergraduate degree in chemistry from the pre-med program at DePauw University in 1958, and MD and pharmacology Ph.D. degrees from Case Western Reserve
University
in 1965. He was an early graduate of the first explicit MD/PhD program which
would later lead to the development of the prestigious Medical Scientist Training
Program. He
then joined the University of Virginia, where he was made
professor in 1970, before moving to Stanford in 1981. Murad left his tenure at Stanford in 1988
for a position at Abbott Laboratories, where he served as a vice
president until starting his own biotechnology company, the Molecular
Geriatrics Corporation, in 1993. The company experienced financial
difficulties, and in 1997 Murad joined the University of Texas Medical
School at Houston to create a new department of integrative biology, pharmacology, and
physiology. Here, he was the Professor and Director Emeritus of The Brown
Foundation Institute of Molecular Medicine for the Prevention of Human Disease
and held the John S. Dunn Distinguished Chair in Physiology and Medicine. In
April 2011, he moved to the George Washington University as a Professor in the
Department of Biochemistry and Molecular Biology.[3]
Murad's key research
demonstrated that nitroglycerin and related drugs worked by
releasing nitric oxide into the body, which acted
as a signaling molecule in the cardiovascular system, making blood vessels dilate. The missing steps in the signaling process
were filled in by Robert F. Furchgott and Louis J. Ignarro of UCLA, for which the three shared the 1998 Nobel Prize
(and for which Murad and Furchgott received the Albert Lasker Award for
Basic Medical Research in 1996). There was some criticism, however, of the Nobel committee's
decision not to award the prize to Salvador Moncada, who had independently
reached the same results as Ignarro.
In May 2012, Municipality of Čair proclaimed him an honorary
citizen. During the ceremony Murad said that all his achievements were
dedicated to his nation, Albania.[4]
Ferid Murad (born September 14, 1936) is an Albanian-American physician and pharmacologist, and a co-winner of the 1998 Nobel Prize in Physiology or
Medicine.
He is also an honorary member of the Academy of Sciences and Arts
of Kosovo.[1]
Contents |
Life
He was born in Whiting, Indiana to Jabir Murat Ejupi, an
Albanian immigrant from Gostivar, Macedonia, and Henrietta Bowman, an
American Christian, Ferid Murad was raised as
a Christian.[2] He received his
undergraduate degree in chemistry from the pre-med program at DePauw University in 1958, and MD and pharmacology Ph.D. degrees from Case Western Reserve
University
in 1965. He was an early graduate of the first explicit MD/PhD program which
would later lead to the development of the prestigious Medical Scientist Training
Program. He
then joined the University of Virginia, where he was made
professor in 1970, before moving to Stanford in 1981. Murad left his tenure at Stanford in 1988
for a position at Abbott Laboratories, where he served as a vice
president until starting his own biotechnology company, the Molecular
Geriatrics Corporation, in 1993. The company experienced financial
difficulties, and in 1997 Murad joined the University of Texas Medical
School at Houston to create a new department of integrative biology, pharmacology, and
physiology. Here, he was the Professor and Director Emeritus of The Brown
Foundation Institute of Molecular Medicine for the Prevention of Human Disease
and held the John S. Dunn Distinguished Chair in Physiology and Medicine. In April
2011, he moved to the George Washington University as a Professor in the
Department of Biochemistry and Molecular Biology.[3]
Murad's key research
demonstrated that nitroglycerin and related drugs worked by
releasing nitric oxide into the body, which acted
as a signaling molecule in the cardiovascular system, making blood vessels dilate. The missing steps in the signaling process
were filled in by Robert F. Furchgott and Louis J. Ignarro of UCLA, for which the three shared the 1998 Nobel Prize
(and for which Murad and Furchgott received the Albert Lasker Award for Basic
Medical Research in 1996). There was some criticism, however, of the Nobel committee's
decision not to award the prize to Salvador Moncada, who had independently
reached the same results as Ignarro.
In May 2012, Municipality of Čair proclaimed him an honorary
citizen. During the ceremony Murad said that all his achievements were
dedicated to his nation, Albania.
Ferid Murad - Biographical
 My father, Jabir Murat Ejupi,
was born in Albania in 1892 and was the oldest of four children. His mother
died when he was 13 years old. He and his family were shepherds and he
subsequently ran away from home to sell candy in the Balkan countries as a
teenager for several years. Although he had less than a year of education, he
learned to speak seven languages before he died at the age of 84 in 1976. He
met a group of other teenagers in Austria and they immigrated to the United
States. The immigration officer at Ellis Island, August, 1913, asked his name,
after which the officer declared him to be John Murad and stamped his papers.
It was not uncommon to have names changed and abbreviated upon immigration.
After working briefly in the steel mills and factories in Cleveland and
Detroit, he settled in Chicago where he had several friends. His career was
quite diverse and although he never admitted it, I learned subsequently from
some of his colleagues that he was quite a playboy with fancy automobiles,
perhaps the reason for my love of nice cars.
My father, Jabir Murat Ejupi,
was born in Albania in 1892 and was the oldest of four children. His mother
died when he was 13 years old. He and his family were shepherds and he
subsequently ran away from home to sell candy in the Balkan countries as a
teenager for several years. Although he had less than a year of education, he
learned to speak seven languages before he died at the age of 84 in 1976. He
met a group of other teenagers in Austria and they immigrated to the United
States. The immigration officer at Ellis Island, August, 1913, asked his name,
after which the officer declared him to be John Murad and stamped his papers.
It was not uncommon to have names changed and abbreviated upon immigration.
After working briefly in the steel mills and factories in Cleveland and
Detroit, he settled in Chicago where he had several friends. His career was
quite diverse and although he never admitted it, I learned subsequently from
some of his colleagues that he was quite a playboy with fancy automobiles,
perhaps the reason for my love of nice cars.
My mother, Henrietta Josephine Bowman, was born in 1918 in Alton, Illinois and
was the third of six surviving children of Elizabeth Lillian and Andrew Orvie
Bowman. My grandmother was a kind and wonderful woman. Only six of her eleven
children survived due to stillbirths and some died of diseases and other
conditions of poverty. My mother went to grade school for several years before
she too quit to help her mother and younger siblings while her mother and two
older sisters went to work. My grandfather was a carpenter who generally worked
part-time and frequently spent his modest paycheck at the local bars before
going home. The childhood poverty of both my parents and their minimal
education did much to influence me and my two younger brothers in our education
and career choices. One brother became a dentist and the other a professor of
anthropology with a PhD degree.
My mother also ran away from home at 17 in 1935 to marry my father who was 39.
I was born September 14, 1936 at home in their hot and small apartment over a
bakery in Whiting, Indiana. My brothers John Abderhaman and Turhon Allen were
born in 1938 and 1944. We were raised in a four room aparttment behind my
parents' restaurant in Whiting, Indiana. This small apartment undoubtedly
influenced my desire for large expensive homes.
The restaurant business had a profound effect on my future and that of my two
brothers. When we were able to stand on a stool to reach the sink we washed
dishes and later when we could see over the counter, we waited tables and
managed the cash register. I did this throughout grade school and high school
each evening and on weekends. I created a game from those chores and learned to
memorize all of the customer's orders in our restaurant with a capacity of 28
customers and before they left I would tally their bills mentally and meet them
at the cash register. I met a diverse and wonderful group of customers that
ranged from laborers in the local refineries and steel mills to local bankers,
businessmen, families and school teachers. My parents worked long hours as is
typical of a family business, particularly a restaurant. My father worked 16 to
18 hours daily while my mother put in similar hours between the restaurant and
raising three children. They owned the building that also included two other
small apartments, another small business and 21 sleeping rooms upstairs. Many
of the tenants were old and retired and my mother would often care for them and
prepare their meals when they were sick. I learned from my mother and
grandmother Bowman about compassion and generosity for people and this in turn
influenced my career choice in medicine. My father taught me some business
skills and how to repair numerous items that were continually breaking down in
this old building. He was quite good at remembering how he took anything apart
in order to repair it and reassemble the pieces as I stood at his side as a
youngster passing him tools.
With this background I knew that I wanted considerable education so I wouldn't
have to work as hard as my parents. Also, I knew at the age of 12 that I was
going to become a doctor. My parents always encouraged us to get an education
and establish a profession. However, my brothers and I grew up with
considerable freedom whether it was saving or spending our tips from the
restaurant or our career choices. This was also applied to our religious
choices as my father was Muslim, my mother Baptist and we were raised in a
Catholic community. Subsequently, my brothers became Catholic when they married
Catholic wives and I was baptized Episcopalian in college. My wife of more than
forty years is Presbyterian, two of our daughters married Jewish men and one
married a Catholic man.
In eighth grade the class was asked to write an essay of our top three career
choices. My choices were 1) physician, 2) teacher and 3) pharmacist (in 1948
clinical pharmacology was not yet a discipline in medicine). Today I do just
that, as I am a board certified physician and internist doing both basic and
clinical research with considerable teaching in medicine, pharmacology and
clinical pharmacology and with a PhD in pharmacology. While I am probably
working much harder and longer hours than my parents, I certainly love my
profession and have considerably more enjoyment and disposable income than they
did. Until my graduation from high school only three of my cousins had finished
high school and no relatives had ever gone to college. Grade school, middle
school and high school were relatively easy for me and with little studying I
was an honor student every semester graduating 5th in my high school class.
Fortunately several high school teachers, some of whom frequented our
restaurant, Jack Taylor in Spanish and history, LaDonna Thue Elson in art,
Bernard Quebeck in music, Jesse Allen in math, and coach Peter Kovachic
convinced me I had some potential and were wonderful counselors and advisers. I
lettered in track and cross country as a distance runner in the one and two
mile events and music. I also played football and basketball but spent most of
my time keeping the bench warm. I played offense and defense left guard at 5'11
" and 140 pounds. After three monsters ran over the top of me I spent more
of my energy with distance running in cross country. While I started to play
golf in grade school, I stopped playing for many years during college and
medical training and I continue to struggle with my game after I began playing
again about 20 years ago.
There was one notable friend since kindergarten, Ronald Delismon, who
influenced me considerably. We competed constantly with everything: grades,
chess, fencing, sports, etc. Today he is an aeronautical engineer recently
retired from Boeing. His projects were always top secret such as the stealth
bomber and some of the star war defense projects. He would never discuss his
work with me for security reasons and often joked with me by saying, "if I
told you, I would then have to kill you". After 57 years we remain the
best of friends and still compete, generally at golf, skiing and more pleasant
encounters. His recent comment was, "one Nobel to zero".
The University of Chicago had a new program in the 1950s that accepted students
after three years of high school and friends in the restaurant who were alumni
from the University of Chicago encouraged me to apply. However, after
considerable thought I decided not to enter college prematurely but rather
completed my senior year in high school. In retrospect, this was the correct
decision for me as my senior year in high school was wonderful. I coasted
through the year with excellent grades and lots of fun participating in the school's
chorus and took the lead in several operettas. This was probably the only year
in school where I wasn't compulsive about grades and didn't study constantly.
Since my parents couldn't afford to help me with my college costs, I looked for
a school that offered the best scholarship. I considered the military programs
at the Naval Academy and Westpoint, but I knew I wouldn't have received the
biology training for medical school since these were primarily engineering
programs with a requisite four years of military duty afterwards. I competed
successfully for a Rector Scholarship at DePauw University in Greencastle,
Indiana, a small and excellent liberal arts university and went there from 1954
to 1958 on a tuition scholarship. The first year my grades were okay but not
great with several A's, one C and the rest B's due to the hazing and
distractions of being a pledge in the fraternity. In subsequent years my grades
progressively improved as I was developing more self confidence and better
study habits. I lived in "annexes", or small apartments with other
fraternity brothers since the fraternity couldn't accommodate all of us and I
generally chose other premeds as roommates. We often studied together and
competed for grades. I was the scholarship chairman of the fraternity and
remained a premed major with a second major in chemistry as I enjoyed both
biology and chemistry. Throughout college I waited tables, taught the anatomy
and embryology labs and worked one and sometimes two jobs during the summers to
cover my expenses. If I had only one summer job I would take additional classes
at one of the local extensions of Indiana University for additional math or
literature classes in order to take more courses in biology, chemistry, physics
or Greek and Latin at DePauw. The Greek and Latin courses in high school and
college were of great value subsequently in learning the root derivatives of
many scientific words.
In the spring of my junior year in 1957 on spring break in Fort Lauderdale,
Florida, I met Carol Ann Leopold, my wife to be. She and her family were from
St. Louis. We were at DePauw together where she was an English and Spanish
major planning to become a teacher. Although she dated many of my fraternity
brothers, I had not met her previously. After spring break we began to date and
I gave her my fraternity pin a month later. Our dates were primarily
"study dates" at the library (the only thing I could afford) and
after mostly A's in my senior year I was elected to Phi Beta Kappa. At
Christmas we were engaged and married within several weeks of graduation from
DePauw on June 21, 1958.
During my senior year of college I began to apply to medical schools and
planned to go to Washington University Medical School in St. Louis. However, my
faculty advisor Forst Fuller, a professor in the biology department and also my
mentor during an elective research project to understand how fish managed
calcium metabolism without parathyroid glands, suggested that I consider a new
MD-PhD program at Western Reserve University. A fraternity brother, Bill
Sutherland, also advised that I consider this new combined degree program that
his father Earl
Sutherland, Jr initiated in Cleveland in 1957. The program paid full
tuition for both degrees and provided a modest stipend of $2000 per year. I
quickly applied and was interviewed on a Saturday morning in February of 1958
by the entire Pharmacology Department. Needless to say, I was awed by the attention
they gave me and decided immediately to accept their offer. Carol, my fiance,
was somewhat concerned that I was now planning seven more years of education
but she has always been understanding and supportive of my training, career
path and numerous moves around the country. The game plan was to have Carol
teach high school English as I went through the combined degree program. These
plans abruptly changed within three months when Carol became pregnant. After
teaching for only one semester, she was asked to resign when the pregnancy
"began to show". Subsequently, she was a substitute teacher, part
time secretary and hospital clinic coordinator as we progressed with our
family; four girls, including a set of identical twins before I finished
medical school and graduate school in 1965. Number five, the first boy, was
born as I finished my residency in 1967. Fortunately, we didn't stop as planned
after number four was born.
As I entered the new combined degree program my mentors were Earl Sutherland, Jr.
the chairman of the Pharmacology Department and Theodore Rall a new young
assistant professor and collaborator of Sutherland's. The year before I arrived
they had discovered cyclic AMP as a "second messenger" of epinephrine
- and glucagon-mediated effects on glycogenolysis in liver preparations. My
assignment was to show that the catecholamine effects on cyclic AMP formation
were due to effects through the beta adrenergic receptor. Alquist had
previously reported that adrenergic effects could be classified as alpha or
beta depending on the relative potency of several catecholamines. The new and
only beta adrenergic receptor antagonist, dichloroisoproterenol, had also been
just described and was to become a useful antagonist in our work. We found that
catecholamine effects on adenylyl cyclase activation in both heart and liver
preparations were, indeed, due to beta adrenergic effects as shown by the
relative potencies of l-isoproterenol, l-epinephrine and l-norepinephrine with
inhibition by dichloroisoproterenol and failure of alpha blockers and agonists
to have effects. I also found that acetylcholine and other cholinergic agents
inhibited adenylyl cyclase preparations, the first description of hormones,
inhibiting cyclic AMP formation. I then became interested in agents that could
block the effects of cyclic AMP on phosphorylase kinase and phosphorylase
activation. This required some novel assays and an acquaintance with numerous
cyclic AMP analogues and other nucleotides including cyclic GMP, cyclic IMP, cyclic
CMP, etc. Many of these nucleotides and their analogues were synthesized by
Theo Pasternak, a professor from Geneva who was on sabbatical collaborating
with Sutherland and Rall. This work subsequently influenced my desire to work
with cyclic GMP as described in my Nobel lecture. Later I again played organic
chemist to make some nucleotides.
I was first in my class every year in medical school and graduate school. This
was a wonderful and exciting time in my life working with these mentors,
watching a new area of biology develop and actively participating in the work.
I loved research as Earl Sutherland was quite a visionary who was able to bring
together multiple disciplines and areas to apply to his work. Ted Rall taught
how to do those fool proof "Sunday experiments" as we came to call
them. It was on Sundays that I could design and conduct those large and complex
experiments with all of Ted's required controls such that the data were
"publishable". We and others in the department were able to determine
that multiple hormones including catecholamines, cholinergics, ACTH,
vasopressin, etc. could increase or decrease adenylyl cyclase activity and
cyclic AMP formation. Prior to this the view of Sutherland was that receptors
and adenylyl cyclase were a single macromolecule or a tightly associated
complex in cell membranes. My work as a student and the work of others
questioned this hypothesis and suggested that different receptors for this
growing list of hormones must be coupled to adenylyl cyclase in yet to be
determined complex ways (see Gilman's and Rodbell's
Nobel lecture of 1994 for a greater description of their subsequent work).
I also enjoyed medical school and found myself learning everything presented
before me. I knew that I couldn't determine what was to be true and important
and many of our faculty acknowledged this as well. Since anything could be
important, I began to learn everything taught. The new experimental integrated
organ-system approach to medical education at Western Reserve permitted me to
assimilate and integrate information more readily. I also thoroughly enjoyed my
clinical rotations in medicine, surgery, OB-GYN, pediatrics, orthopedics,
neurology, etc. There were few clinical rotations that I didn't think about as
a possible discipline for my future academic career. I subsequently learned
that I was at the top of the medical school and graduate school class each year
and received prizes at graduation for both clinical medicine and research. I
was in my element and loved it. There was no doubt in my mind about an academic
career in medicine, research and teaching.
In order to supplement my stipend with so many children, I moonlighted at the
Cleveland Clinic working one or two nights per week on the OB-GYN service to
follow mothers with pelvic exams as they progressed through labor, assisted in
deliveries and Caesarian sections and then scrubbed tables and floors after
each delivery. All of this for $20.00 per night for 12 hours of work from 7:00
p.m. to 7:00 a.m. one or two nights per week for four years. On slow evenings I
was able to study, analyze lab data and write research protocols. Some nights
required that I work all night and then attend a full day of classes the next
day. I continued this during my clinical clerkships requiring my absence from
my family as often as 4 to 5 nights per week. However, I tried to have dinner
with my family as often as my schedule permitted. My wife and children were
very understanding. They grew up as wonderful children and adults in spite of
my absence, obviously due to a devoted wife and mother. My current fetish is my
5 grandchildren who I try to spend as much time with as possible, undoubtedly
due to my guilt as an absent father. I did manage to spend several weeks each
summer with my family as we took them camping all over the U.S. to various
scientific meetings. There are only a few states where we have not camped
together as a family and they all became proficient swimmers at a young age.
I decided to go to Massachusetts General Hospital for my internship and
residency in medicine (1965-67). What a wonderful experience this was with some
of the worlds' leading scientists, teachers and clinicians. Our group of 14
housestaff included exciting bright minds such as Tom Smith, Tony Gotto, Jim
Willerson, Ed Scolnik and others that had considerable influence on me. My attendings
and chief residents included Alex Leaf, Dan Federman, Roman DeSanctis, Frank
Austen, Sam Thier, Ken Shine and others. As a resident Joe Goldstein and Mike
Brown were two of our interns. I couldn't have asked for a greater introduction
to medicine in spite of being on call every other night and weekend. I did,
however, miss the laboratory and each spring I found myself in the library
reading many of the abstracts of the Federation meeting (currently FASEB
meeting) to see what I was missing in "second messengers and hormone
signaling". I generated a notebook that contained numerous "obvious
experiments" to be done. When I subsequently went to NIH as a clinical
associate in the Heart Institute I was able to do many of the planned experiments
in Martha Vaughan's laboratory. She too was an excellent mentor with a style
different from either Sutherland or Rall. She gave me considerable freedom to
pursue a number of areas related to cyclic AMP and hormonal regulation. Her
husband, the late Jack Orloff, while superficially a gruff and tough man, was a
sensitive person and talented scientist. I was indeed fortunate that they and
many others at NIH influenced my thinking and career planning. I soon learned
that I had numerous role models and attempted to extract the best features of
each as I planned my career path and future.
I remained at NIH for more than three years (1967-70) when the University of
Virginia called to recruit me to develop a new Clinical Pharmacology Division
in the Department of Medicine with an appointment as an Associate Professor in
medicine and pharmacology. I couldn't resist the offer from Ed Hook, the new
chairman of medicine and Joe Larner, the new chairman of pharmacology. Other
faculty such as Tom Hunter, the Vice President of Medical Affairs, Ken Crispell
the Dean, Bob Berne, Bob Haynes and others influenced my decision to leave NIH.
I had known Larner, Berne and Haynes since they were faculty at Western Reserve
when I was a student. Charlottesville was also an appealing place to raise my
five children. Some colleagues around the country, particularly David Kipnis,
another one of my role models, questioned me about going to Charlottesville.
Just the previous year I called him to apply for a fellowship in endocrinology
at Washington University. I was then 33 years old with 5 children and his
advice was appropriate. He said, "Fred, time for you to get a job and
support your family", and I took his advice to heart.
I joined the faculty at the University of Virginia, September 1, 1970 and
nervously thought about how I could launch my own independent research career.
I decided to work with cyclic GMP as it was beginning to emerge as a possible
new "second messenger" to mediate hormone effects. This is detailed
in my Nobel lecture. I remained at the University of Virginia from 1970 to 1981
where I was promoted as one of the youngest professors in 1975; I was also
asked to become the Director of their Clinical Research Center in 1971 and the
Director of Clinical Pharmacology in 1973. I built a research program with both
clinical and basic studies and started to recruit many exciting students and
fellows to work with me. Of the 82 fellows and students I have trained and
collaborated with to date twenty are professors, chairmen, research directors
and division chiefs around the world. I view them as offspring and keep in
contact with most of them in my travels. There is no question that one of my
greatest accomplishments is to have participated in the training of such
successful scientists in my own laboratory and also influenced the careers of
many talented medical students, graduate students and housestaff.
After looking at many university positions around the country as a chair of
medicine or pharmacology and industrial positions, I decided to go to Stanford
in July 1981 as Chief of Medicine of the Palo Alto Veterans Hospital, a
Stanford affiliated hospital. I was a professor of medicine and pharmacology
and the associate chairman of medicine. While it was difficult to leave many
friends and colleagues at the University of Virginia where we conducted the
first experiments with the biological effects of nitric oxide, I couldn't turn
down this exciting opportunity at Stanford. Ken Melmon was chairman of medicine
and during our first three years together we recruited about 30 new young
faculty. Inspite of the large administrative and clinical teaching demands, I
continued to supervise a large and productive laboratory with about 15
students, fellows and staff. Trainees continued to come to our laboratory from
all over the world. Some of my students and fellows subsequently went to
medical school and after completing residencies have become very productive
physician scientists at a number of institutions.
After a stint as Acting Chairman of Medicine at Stanford (1986-88), I left to
become a Vice President at Abbott Laboratories as I was becoming concerned
about managed health care on the horizon and its possible effects on patient
care, research and education. After considering several industrial positions, I
chose Abbott primarily because of its president Jack Schuler, a sales and
marketing person with an MBA from Stanford who also had considerable vision. We
worked well together as he taught me many business principles and I taught him
about drug discovery and development. I enjoyed the access to all of Abbott's
resources, scientific staff, instrumentation and what initially seemed like an
unlimited research budget. I eventually learned that one can never have enough
resources when one looks for novel therapies of major diseases; it's an
expensive undertaking. Nevertheless, in four years of directing their
pharmaceutical discovery and development programs we were able to discover many
novel drug targets and we brought forward about 24 new compounds for clinical
trials for various diseases. I continued to have a very productive lab with two
NIH grants, some outside funding for fellows and about 20 scientists working
with me on nitric oxide and cyclic GMP. The administrative demands and travel
were considerable since I was a corporate officer, vice president and also
overseeing many industrial collaborations around the world. When I left Abbott
I was supervising about 1500 scientists and staff and probably earned the
equivalent of an MBA from the experience on the job plus periodic management
courses required by the company. Before my arrival at Abbott the company had no
postdoctoral fellows or extramural funding. When I left we had about $3.5 mill.
per year of extramural grant support and about 35 fellows in pharmaceutical
research. Unfortunately, Abbott reorganized its senior management and my
business role models were asked to leave. As Abbott's senior scientist I found
myself wedged between upper management, the marketing staff and the scientists
and constantly was defending my decisions about the research programs. There
were always considerable marketing pressures on me that in my opinion were
often the wrong decisions to develop novel therapeutics for diseases without
adequate therapy.
I left Abbott in 1993 to be a founder, President and CEO of a new biotech
company, Molecular Geriatrics Corporation. The plan was to create another
intensive research-based biotech company. Unfortunately, my investment banker
never raised the amounts of money promised and he eventually lost a major
personal fortune with his leveraging tactics. I found myself skipping around
the world to find investors and partners to keep the company afloat and pay the
bills. After a partnership with a major pharmaceutical company and some more
financing as a private company, I left to rejoin academics, hopefully much
wiser.
After considering a number of Vice President, Dean positions and Chairmanships,
I realized that such positions would probably totally remove me from the
laboratory, fellows and students, things I could not give up. In April 1997, I
became the University of Texas-Houston's first chairman of a newly combined
basic science department, Integrative Biology, Pharmacology and Physiology. I
am also creating a new Division of Clinical Pharmacology jointly between our
department and medicine. I plan to continue an active basic and clinical
research program and will participate in clinical medicine and teaching again.
Thus, I have come full circle. I am back in my academic element again and I
love it. I also expect to continue some business adventures and exercise my
entrepreneurial skills, areas that I also enjoy and view as lucrative hobbies.
The freedom and intellectual environment of academic medicine and bright young
students and fellows are exciting and a daily joy for me. After all, I hope to
tell Ron Delismon some day "Two Nobels to zero".
From Les Prix
Nobel. The Nobel Prizes 1998, Editor Tore Frngsmyr, [Nobel
Foundation], Stockholm, 1999
This
autobiography/biography was written at the time of the award and later
published in the book series Les Prix
Nobel/Nobel
Lectures. The information
is sometimes updated with an addendum submitted by the Laureate.
Copyright © The Nobel Foundation 1998
Addendum,
September 2005
It has
been seven years since receiving the Nobel Prize for Physiology or Medicine for
my work with nitric oxide and cyclic GMP. Life has been extremely busy. I have
continued as chairman of the Department of Integrative Biology and Pharmacology
at the University of Texas - Houston. I have expanded the department with the
recruitment of six new, young, faculty, but some retirements and one death in
the department kept us about the same size until some of the Dental School
faculty joined us to consolidate some of the Dental School.
My
laboratory has been very active with about 15 to 18 scientists which is our
usual size for the past 25 years. We have found ourselves redirecting some of
our research interest with nitric oxide and cyclic GMP into some new directions
to maintain our lead in the field and address new challenging questions with
soluble guanylyl cyclase regulation, and the role of nitric oxide and cyclic
GMP in mouse and human embryonic stem cell proliferation and differentiation.
While
research grant applications and support are always a nervous and time consuming
process, several foundations and donors have generously supported our work and
provided me with a handsomely endowed chair. These flexible and discretionary
research funds have been most appreciated to pursue some of our research ideas,
or accept another outstanding young scientist and trainee.
The
academic world like the business world is busily involved with layers of review
and compliance. With about one-half of the number of scientists in our
department that I had as Chairman of Medicine at Stanford University the paper
work has probably tripled. The developers of e-mail should be admonished for
destroying so much paper and trees and wasting hours and hours of my time. It
seems that everyone in the University feels obliged to send me all of their
email copies, often after four pages of addresses, followed by a brief useless
message. Perhaps all employees should be allocated some annual allotment of
emails which, if exceeded, results in salary reductions.
Shortly
after the Nobel Prize, I was asked to become the Director of our Institute of
Molecular Medicine which I also accepted. For the past eight years I have held
two senior positions in the University, as Chairman of the Department and
Director of the Institute, each normally a full-time position. While at the
University of Virginia, Stanford University, and Abbott Laboratories I also
held two positions simultaneously. This is perhaps due to my workaholic
tendencies.
Being
the Director of the Institute has also provided me with a significant building
and recruiting opportunity. I was able to convince the University President to
engage in a major fund raising campaign of $200 million. About half was used to
build a new research building, of about 230,000 sq ft for the Institute, and
the other half to recruit new faculty and scientists. The state of Texas, the
Houston community, and local foundations have been most generous and we will be
moving into our new research building in mid-2006. We expect to recruit 30 to
40 new faculty over the next three to five years, plus their research staff and
trainees. We expect to triple our current number of scientists.
A very
time consuming activity in the past seven years has been my travel and
lecturing. I have visited about 35 to 40 countries during the past seven years
and traveled about 100,000 to 150,000 miles per year. I am invited to all sorts
of meetings and functions around the world to dedicate buildings, hospitals,
participate in conferences, scientific meetings, university seminars, consult
for companies and governments, etc ... Presumably, it is assumed that by having
received the Nobel Prize you are automatically an expert on all topics, fields
and disciplines. I have even been invited on panels of Nobel Laureates to
discuss methods to promote peace and education around the world. While
participating in these many travels and meetings, I have also declined many
invitations because time does not permit the travel or because of conflicts in
scheduling. After all, I do have a day job and must be home occasionally to
pick up my paycheck. I have had many memorable experiences and meetings and
fortunately my wife, Carol, accompanies me on much of my travel. I have had
meetings with Palestine's Chairman Yasser Arafat, Israel's Prime Minister
Netanyahu, Presidents Lee and Chen of Taiwan, Chief Executive Tung of Hong
Kong, President Medani of Albania, President Trajkovski of Macedonia, Premier
Wen Jiabao of China, President Clinton, President Bush, many congressman and
senators, governors and mayors.
I have
also met dozens of Nobel Laureates and attended conferences and meetings with
them. Carol and I have become friends of many Laureates and their spouses and
many travel and lecture as much as I do. For those who are retired, it hasn't
been quite so demanding or difficult.
My
office and home are filled with artifacts, photographs, plaques, medals,
statues, gifts and memorabilia. You receive numerous honorary degrees and
certificates to wall paper your office at both work and home. We have run out
of wall and surface space in my office and home and have begun to create piles
to organize in the future. Since I have no plans for retirement, my children
and grandchildren will probably have to organize the materials some day. One of
the more humorous and memorable events was being grand marshall of the fourth
of July Parade, with my wife and some of our grandchildren on the float, in my
home town Whiting, Indiana. I have also given lectures to children in schools,
churches and mosques. After a number of such requests, I prepared a children's
educational video that can be viewed on the Nobel website that discusses the
Nobel Prize and nitric oxide.
On one
of my several trips to Macedonia, my father's homeland, Carol and I arranged
for one of our daughters to adopt a three-month-old Albanian baby girl. The
trip, at our expense, required that I give several lectures and meet with many
dignitaries. I consider this one of my best honorariums.
The
Nobel Prize has also influenced my grandchildren who have been asked to discuss
nitric oxide and the Nobel Prize in their classes after a classmate's new
premature sibling required inhaled nitric oxide for pulmonary hypertension.
Press conferences with the media and radio, and television interviews are
frequent. The media often wants to talk about Viagra, while I attempt to lead
them into more medically significant areas: such as, pulmonary hypertension in
premature babies, wound healing, endothelial dysfunction with atherosclerosis,
hypertension, or diabetes, where nitric oxide can be much more important
medically.
While
the Nobel Prize ceremonies in 1998 in Stockholm were quite a treat, the 100th
anniversary Nobel Reunion in 2001 allowed me the opportunity to participate in
the ceremonies and festivities, again, with less anxiety and an opportunity to
absorb and savor the activities and functions. Life after the Nobel Prize is
quite exciting, interesting and also demanding. I thought the attention and
notoriety would subside within several months after receiving the Prize.
However, there is no indication that this is the case seven years later.
Wherever you go you can't escape the media and the attention. The numerous
invitations to travel, lecture, attend conferences, consult for governments,
universities, and companies have not subsided. It is exciting, rewarding,
educational, lucrative and exhausting. You can rarely let your guard down and
hide or relax. You don't dare pick your nose or scratch in some places for fear
that someone will catch you on camera or video. When you travel, you often feel
like you are on "Candid Camera".
Although
I receive multiple faxes, phone calls and FedEx's when I travel, when I return
there are stacks of correspondences and long lab meetings with my staff to
review our research progress before preparing for the next trip.
Robert Francis Furchgott (June
4, 1916 – May 19, 2009) was a Nobel Prize-winning
American biochemist.

Contents
|
Life and career
Furchgott
was born in Charleston, South Carolina, to Arthur Furchgott (December 1884 -
January 1971), a department store owner, and Pena (Sorentrue) Furchgott. He
graduated with a degree in chemistry in 1937 from the University
of North Carolina at Chapel Hill, received his Ph.D in
biochemistry at Northwestern
University in 1940. He was faculty member of Washington
University School of Medicine from 1949 to 1956. From 1956
to 2009, he was professor of pharmacology at the
State
University of New York Downstate Medical Center.
In
1978, Furchgott discovered a substance in endothelial cells that
relaxes blood
vessels, calling it endothelium-derived
relaxing factor (EDRF). By 1986, he had worked out EDRF's nature
and mechanism of action, and determined that EDRF was in fact nitric oxide (NO),
an important compound in many aspects of cardiovascular physiology. This
research was important in explaining the action of Viagra,
treatment of blue babies, and other medical and health-related issues.
In
addition to the Nobel
Prize in Physiology or Medicine he shared in 1998 (with Louis Ignarro and Ferid Murad),
Furchgott also received a Gairdner
Foundation International Award for his groundbreaking
discoveries (1991) and the Albert
Lasker Award for Basic Medical Research (1996), the latter also with Ferid Murad.
Furchgott,
who was Jewish,[1] lived
for most of his married and career life in Woodmere, NY (Long Island). He was
married to Lenore Mandelbaum (February 1915 - April 1983)[2] from
1941 until she died aged 68. They had three daughters: Jane, Susan and Terry.
His daughter, Susan, was a prolific artist in the San Francisco counter culture
and a co-founder of the Kerista Commune (she
was also known as "Even Eve" and "Eve Furchgott"). Robert
Furchgott later married Margaret Gallagher Roth, who died March 14, 2006.[3] He
served as a professor emeritus at the State
University of New York Downstate Medical Center. In
2008 he moved to Seattle's Ravenna neighborhood. Furchgott died on May 19, 2009[4] in
Seattle. He is survived by his three daughters, four grandchildren, and one
great-grandchild.
See also
References
|
|
This article includes a list
of references, but its sources remain unclear because it has
insufficient inline citations. improveintroducing (May 2009) |
- Raju, T N (2000), "The Nobel chronicles.
1998: Robert Francis Furchgott (b 1911), Louis J Ignarro (b 1941), and
Ferid Murad (b 1936).", Lancet
(2000 Jul 22) 356 (9226): 346, PMID 11071225
- Rabelink, A J (1998), "Nobel prize in
Medicine and Physiology 1998 for the discovery of the role of nitric oxide
as a signalling molecule", Nederlands
tijdschrift voor geneeskunde (1998 Dec 26) 142 (52): 2828–30, PMID 10065255
- Laufs, U; Erdmann, E (1998), "Nitric
oxide as a signal molecule in the cardiovascular system. Nobel Prize for
Medicine in 1998", Dtsch. Med.
Wochenschr. (1998 Dec 18) 123
(51–52): 1562–5, doi:10.1055/s-0029-1237297, PMID 9893684
- Hansson, G K; Jrnvall, H; Lindahl, S G (1998),
"The Nobel Prize 1998 in physiology or medicine. Nitrogen oxide as a
signal molecule in the cardiovascular system", Ugeskr. Laeg. (1998 Dec 21) 160 (52): 7571–8, PMID 9889673
- Nielsen, T T; S¿rensen, K E (1998),
"Discovery of "endogenous nitroglycerin", NO, as cellular
signal molecule", Ugeskr. Laeg.
(1998 Dec 21) 160 (52): 7567, PMID 9889670
- Mitka, M (1998), "1998 Nobel Prize
winners are announced: three discoverers of nitric oxide activity", JAMA (1998 Nov 18) 280
(19): 1648, doi:10.1001/jama.280.19.1648, PMID 9831980
- Hansson, G K; Jrnvall, H; Lindahl, S G
(1998), "1998 Nobel Prize in physiology or medicine. Nitric oxide as
a signal molecule in the cardiovascular system", Lakartidningen (1998 Oct 21) 95 (43): 4703–8, PMID 9821753
- Furchgott, R F (1996), "The 1996 Albert Lasker
Medical Research Awards. The discovery of endothelium-derived relaxing
factor and its importance in the identification of nitric oxide", JAMA (1996 Oct 9) 276
(14): 1186–8, doi:10.1001/jama.276.14.1186, PMID 8827976
1.
^ American Jewish Recipients of
the Nobel Prize
2.
^ Social
Security Death Index
3.
^ http://query.nytimes.com/gst/fullpage.html?res=9800E1DB143AF934A25750C0A9609C8B63 The New York Times
![]()
![]()
![]()
was
born in the lovely coastal city of Charleston, S.C. in 1916 and lived there
until I was thirteen. In Charleston I first became enamored of "natural
history" when I attended nature study classes and field trips to nearby
beaches, marshes and woods, sponsored by the Charleston Museum. I became an
avid shell collector and bird watcher (that was before the term
"birder" was coined), and I still enjoy these hobbies. In 1929, my
family moved from Charleston to Orangeburg, S.C., an inland, rural town of
about 8,000 inhabitants, where my mother had grown up and still had some
family. The reason for the move was that the Furchgott department store in
Charleston, which had been started by my grandfather and was being run by my
father and his two brothers, was unable to survive in the midst of the
Depression, and my father decided to open a women's clothing store in
Orangeburg. So I spent my high school years in Orangeburg, enjoying small town
life and competing with my first cousin Edwin Moseley for the highest grades in
our class. He won.
Within the first couple of years of high school, I knew that I would like to be
a scientist. My parents were encouraging: they gave me chemistry sets and a
small microscope as presents. I liked to read popular books about scientists,
although there were not many available at that time. My father subscribed to
the Sunday New York Times, in which there was often a column on science that I
found very exciting.
During the four years that I was in high school, my older brother Arthur was at
the University of North Carolina at Chapel Hill. I wanted to attend college
there also, but that was not possible when I finished high school in 1933
because tuition for me, as an out-of-state resident, was more than my father
could afford at that time. So I spent my freshman year at the University of
South Carolina, where my tuition was much less. However, by the summer of 1934,
my father moved his business from Orangeburg to Goldsboro, N.C., where he felt
that the local economy was better. So now, as a resident of North Carolina, I
was able to register at the University at Chapel Hill as a sophomore majoring
in chemistry.
At Chapel Hill, I had a number of excellent teachers in chemistry. During my
junior and senior years, I had a small amount of financial support from an NYA
job (NYA being the initials of the National Youth Administration set up by the
federal government to help students during the Depression). In that job, I was
a lab assistant in research to a junior faculty member working on the physical
chemistry of solutions of cellulose. I had decided early in my college years
that I would go on to graduate work in some branch of chemistry. My preference
by the time I was a senior was physical organic chemistry. I sent letters to
dozens of chemistry departments applying for a graduate fellowship or teaching
assistantship. I had an excellent academic record, but by graduation time I
still had no definite offer of a position for graduate training. I was almost
resigned to taking a job in chemical industry, when around the middle of June
while I was in Florence, S.C., where my parents now lived, an unexpected offer
of a teaching assistantship came to me from the Physiological Chemistry
Department of Northwestern University Medical School in Chicago. I was to be a
graduate student of Dr Henry Bull, who had recently come to Northwestern, and
whose research interests were physical chemical aspects of biochemistry.
Northwestern and Cold Spring Harbor (1937-1940)
Before I went to Chicago, I worked for two summer months in 1937 for Eastern
Airlines at the Philadelphia airport - a job which my older brother Arthur, who
was employed by that airline, helped me obtain. The job allowed me to save some
money and also allowed me free air travel to Chicago. That helped a lot since
my stipend as a teaching assistant at Northwestern was only $50 a month for a
nine-month academic year. When I arrived in Chicago, it had already been
arranged for me to share a room with two more advanced graduate students.
Living in Chicago was quite a change from living in the Carolinas. When I would
walk to work in the winter from our rooming house, which was about a mile from
the medical school, the chill wind whipping in from Lake Michigan along Chicago
Avenue was quite an experience for a Southern boy.
My course work at Northwestern was partly at the medical school, and partly at
the Evanston campus to which I would travel via the El. At the Evanston campus,
my courses were mainly in physical chemistry under Dr Malcolm Dole, who was
also on my PhD advisory committee. At the Chicago campus, I had to take
physiology and bacteriology (along with medical students), Henry Bull's course
on physical chemistry in biochemistry, and some assorted graduate courses in
physiology and biochemistry. The physiology course was under the direction of
Dr Andrew Ivy, who had built up a sizeable physiology department faculty for
those times. In contrast, the biochemistry faculty consisted only of the
chairman, Dr Chester Farmer, Dr Bull and two part-time lecturers.
My laboratory work with Bull started out with the preparation of purified egg
albumin. He was studying physical chemical changes in this protein after
different methods of denaturation. He had begun to involve me in some of his
studies when the summer of 1938 came along, and that turned out to be a special
summer for me. Bull had been invited to present a paper on his work at the
sixth Cold Spring Harbor Symposium on Quantitative Biology which was to take
place at the Cold Spring Harbor Biological Laboratory of the Long Island
Biological Association. The theme of the symposium, which was to run for five
weeks in a leisurely fashion was the structure and function of proteins. Bull
had obtained permission from the director of the Cold Spring Harbor Laboratory,
Dr Eric Ponder, for me to attend the symposium, while earning my room and board
by running the lantern slide projector at the lectures. The symposium was very
exciting. I met many distinguished scientists. Ponder and a
physician-scientist, Harold Abramson, arranged to have me assist in a research
project at the laboratory for the rest of the summer after the symposium was
over. The project was on the electrophoretic mobility of rabbit erythrocytes
and ghosts, measured with the use of a microelectrophoresis cell and light- and
dark-field microscopy.
By the end of the summer, I had become very interested in the physical
chemistry of the red blood cell membrane. When I returned to Northwestern in
the fall of 1938, Bull approved continuation of my research on red blood cells
as a PhD thesis project. In particular, I was fascinated by the unexplained
phenomenon of the transformation of mammalian red blood cells, suspended in
unbuffered isotonic saline from discs to perfect spheres when a small drop of
the suspension was placed between slide and coverglass. I discovered that the
disc-sphere transformation depended on two factors. The first was a rise in pH
to over 9.0 in the unbuffered suspension, as a result of the alkaline nature of
the glass surfaces (pH being measured with a semi-micro glass electrode that I
constructed). The second factor was the removal from the suspension of the red
blood cells by adsorption onto the glass surfaces of the slide and coverglass
of a substance in the suspension that prevented sphering on elevation of pH of
the suspension. I demonstrated that this substance, which I termed the
anti-sphering factor, was serum albumin which could not be effectively removed
from the red cells simply by multiple washing and centrifuging. In addition to
the work on shape changes in erythrocytes, my PhD thesis work involved
additional studies on the electrophoresis of the cells under various conditions
and on other aspects of the physical chemistry of erythrocyte membranes.
In the summer of 1939 at the invitation of Ponder, with whom I had extensive
correspondence during the year and who had become in effect the major advisor
for my PhD thesis research, I returned to Cold Spring Harbor to continue
research on red blood cells. To earn my room and board, I waited on tables in
the communal dining room. I also was able to attend the symposium talks of that
year, which were on the subject of biological oxidations. There I first became
aware of the new developments in oxidative energy metabolism and the importance
of high energy phosphate compounds. Among the many outstanding biochemists
attending were L. Michaelis, Fritz
Lipmann and Carl
Cori. Ponder and his young wife Ruth were very hospitable to
me. I was much impressed with his skill in applying mathematics in his
research, his facility in scientific writing, and his large collection of
records of classical music.
I was able to complete and defend my thesis in time to receive the Ph.D. degree
in June of 1940. Earlier that spring I had attended the annual meeting of the
Federation of American Societies for Experimental Biology (FASEB) in New
Orleans. I had fortunately been asked by Henry Tauber, an Austrian biochemist
working for a pharmaceutical firm in Chicago, to share the driving in his car
on the round trip to New Orleans as well as his room in a rundown hotel in New
Orleans. Thus, I was able to attend this meeting at very little expense. At the
FASEB meeting in New Orleans, where gatherings of participants were still
called "smokers" and even a fancy meal was not more than two dollars,
I had some interviews with persons about possible post-doctoral jobs. One of
the interviews was with Dr. Ephraim Shorr, an Associate Professor of Medicine
at Cornell University Medical School in New York City, whom I had met at Cold
Spring Harbor the summer before. A few weeks later Shorr offered me a
postdoctoral position in his laboratory. Although I was hoping to get a
position which would allow me to continue work on physical chemistry of
proteins or cell membranes, none came through, and I accepted the position with
Shorr, with the understanding that I would begin in September.
The reason for waiting until September to begin work at Cornell was because I
wanted to spend one more summer at the Biological Laboratory at Cold Spring
Harbor. This time, however, I went there as an invited speaker at the symposium
which that summer was on the topic of permeability of cell membranes. My talk
was entitled "Observations on the structure of red cell ghosts." At
that symposium, there were again a number of established distinguished
scientists like K.S. Cole, Robert Chambers and F.O. Schmitt; and in addition, a
number of bright young scientists like Hans Neurath, who had also been at the
1938 symposium, Hugh Davson, who with Danielli had developed the lipid bilayer
membrane model, and Benjamin Zweifach, with whom I was to collaborate later in
research.
Cornell University Medical College (1940-1949)
I stayed at Cornell University Medical College working in the laboratory of'
Ephraim Shorr for nine years. When I arrived, Sam Barker, a young research
associate, was there to instruct me in methods and procedures they were using
to study tissue metabolism (largely using Warburg manometers) and the turnover
of rather ill-defined tissue organic phosphate fractions from canine cardiac
muscle during incubations in vitro.
For such studies the lab was one of the first to use radioactive phosphate,
which we obtained from the cyclotron laboratory at Berkeley. Barker left toward
the end of my first year at Cornell; and I was then responsible for running the
laboratory for Shorr. Shorr himself, would sometimes take part in preparing
tissue for the Warburg experiments. He was quite capable in the laboratory in
addition to being a busy and excellent clinician.
During my first two years at Cornell, my major project was on phosphate
exchange and turnover, using radioactive phosphate and slices of dog left
ventricular muscle. A full paper on the work was published in the journal of
Biological Chemistry in 1943. The methods and equipment we used in that work
have long been superseded, but we did manage with chemical and some early
enzymatic methods to show the extremely fast turnover of creatinine phosphate
and the terminal phosphate of ATP in resting cardiac muscle.
The 1943 paper was my first full publication after three years of work at
Cornell. One likely reason for sparse output was that the United States had
entered World War II in December of 1941, and Shorr, like many others, began to
undertake research that had more relevance to the war effort. With government
and other support, he shifted the major research in the lab to circulatory
shock - first on changes in tissue energy metabolism resulting from hypoxia associated
with hemorrhagic shock, and then mainly on factors that might account for
"irreversible" shock, the condition in which restoration of blood
volume is no longer able to raise pressure and sustain life in the animal
subjected to maintained low blood pressure as a result of controlled
hemorrhage. To help in this new line of research, Shorr recruited Benjamin
Zweifach, then a bright young physiologist who had trained with Robert Chambers
and had developed a beautiful method for microscopic observation of blood flow
in part of the mesentery (the "mesoappendix" area) of the
anesthetized rat. In brief, the "rat mesoappendix test", conducted by
Zweifach and technicians whom he trained, produced evidence by 1944 for two
vasoactive factors in circulatory shock. The first factor appeared in the
plasma of dogs in the early reversible (by transfusion) stage of hemorrhage.
Intravenous injections of this plasma increased the sensitivity of the small
arterioles and pre-capillary sphincters to topically applied epinephrine in the
mesoappendix test. This factor was termed VEM (for vasoexcitatory materials).
As the irreversible stage of circulatory shock developed, VEM activity
disappeared from the plasma and a new factor appeared which markedly decreased
the sensitivity to epinephrine in the mesoappendix test. This factor was termed
VDM (for vasodepressor material). We developed evidence, in part from in vitro experiments with tissue slices,
that hypoxic kidney was the probable source of VEM and that hypoxic liver was
the probable source of VDM. By late 1945, these developments led to a lead
article in the journal Science by
Shorr, Zweifach and myself.
During the war years, I was not solely involved in research on tissue
metabolism and circulatory shock. In 1943, Eugene DuBois, chairman of the
Department of Physiology at Cornell, arranged that I join his department as an
instructor in order to replace a staff member lost to military service.
Although I was teaching in physiology, I still spent most of my time in
research in Shorr's lab, which was partially funded by the federal Office of
Scientific Research and Development. The work on VEM and VDM continued after
the war ended. I had attempted to isolate the VEM-like material that
accumulated in incubation fluid when kidney slices were incubated
anaerobically. I was able to concentrate it somewhat and it appeared to be a
labile dialyzable peptide, but I failed to isolate it. On the other hand,
Abraham Mazur, a professor of biochemistry at the City College of New York who
worked part time with us, purified a VDM-like material from liver which
appeared to be ferritin. (Ferritin or not, we might now wonder whether VDM
could somehow be related to nitric oxide!)
Unfortunately, the only bioassay procedure for detecting VEM and VDM activity
was that involving changes in sensitivity to epinephrine in the rat
mesoappendix test. Intravenous injections of solutions containing high levels
of impure VEM or purified ferritin did not effect blood pressure in
experimental animals. Attempts to develop an in vitro bioassay system also failed. These failures tempered my
enthusiasm, and I think that of Zweifach, for the significance of VEM and VDM
in the regulation of circulation. However, the failed attempts to develop an in vitro bioassay for VEM and VDM were
very important for me for they introduced me to the pharmacology of smooth
muscle, a subject that has been a major interest of mine ever since.
Two of the isolated smooth muscle preparations that I unsuccessfully tested for
bioassay of VEM and VDM were a helically-cut strip of rabbit aorta, which
responded with contraction to epinephrine, and a longitudinal segment of rabbit
duodenum, which exhibited spontaneous rhythmic contractions that were inhibited
by epinephrine and stimulated by acetylcholine. At that time, contractions of
such smooth muscle preparations mounted in organ baths were recorded with
isotonic levers on kymographs. One day in the course of making tests on
segments of rabbit duodenum mounted in oxygenated Krebs solution, I was
surprised to see that during the first hours of the experiment, contraction
amplitude did not stabilize as usual but declined gradually and markedly even
though the rhythmic frequency remained unchanged. I suspected that my
technician had forgotten to add glucose to the Krebs solution. Adding glucose
now quickly increased contraction amplitude to the normal level. This finding
led to a simple procedure for finding out what sugars and fatty acids could be
utilized for energy for contraction in the intestinal smooth muscle under
aerobic and anaerobic conditions and to analyze the sites of action of
metabolic inhibitors.
In the spring of 1949, 1 had two interesting offers at the assistant
professorship level - one in physiology at Duke and one in pharmacology at
Washington University School of Medicine. I decided on Washington University,
partly because the new chairman there, Oliver Lowry, was someone I had known in
the Enzyme Club in New York City and partly because I had begun to be very
interested in pharmacology as a discipline. This was partly because of the
studies I had begun on the effects of drugs and other agents on smooth muscle
preparations in vitro, but also in
large part because of my close friendship with Walter Riker, who was then a
junior member in the Pharmacology Department at Cornell at the beginning of a
distinguished career. His enthusiasm for research in pharmacology was
contagious.
In the summer of 1949, my family and I drove from New York to St. Louis. My
wife, Lenore, a native New Yorker, said she felt like she was going West in a
covered wagon. By that time we had two daughters, ages four and one. Later we
had a third daughter born in St. Louis. It might be noted here that none of my
daughters became scientists. Instead, they all went into art (like my younger
brother, Max). It might also be noted here that my wife Lenore died in 1983;
and that now I have a new wife, Margaret (Maggie). I have been very fortunate
in having wives who encouraged my work, even though it often reduced the time I
could give to family matters.
Washington University (1949-1956)
My seven years in the Pharmacology Department at Washington University were
enjoyable ones. Oliver (Ollie) Lowry had been appointed chairman of that
department a year or so before I came. He was already well recognized for his
ingenuous methods involving enzymology , spectrometry and fluorometry in the
quantitative analysis of important enzymes, substrates and products in
extremely small amounts of tissue. He was very helpful in introducing me to
enzymatic-spectroscopic methods (as developed by kalckar) for analysis of ATP,
ADP and AMP. As a new chairman, Lowry inherited two faculty members, Helen
Graham and Edward Hunter, and recruited two new ones, namely myself and Morris
(Morrie) Friedkin. I had never had a course in pharmacology as a student, much
less taught in one, and so I had to spend a lot of time during my first year in
St. Louis keeping ahead of the medical students. Later, when I set up my own
department in Brooklyn, I adopted for the pharmacology course there much of the
lecture, laboratory and conference program that I had participated in at St.
Louis.
Lowry's department was a stimulating place for research. Over the years I was
there, the departmental staff grew steadily. Lowry attracted outstanding
postdoctoral fellows, such as Eli Robbins and Jack Strominger. We often joined
the members of Carl Cori's Biochemistry Department for seminars and journal
club meetings.
My first research project at Washington University was a continuation of the
work I had begun at Cornell on energy-metabolism and function of rabbit
intestinal smooth muscle. I was able to obtain a small grant to support my
research on smooth muscle, and to hire a technician, Marilyn (Wales) McCaman,
who later became my first graduate student. By the middle of 1951, my favorite in vitro smooth muscle preparation had
shifted from the rabbit duodenum to the rabbit thoracic aorta. I had found that
the helical (spiral) strip of that vessel, properly cut and mounted in organ
chambers for isotonic recording, gave very reproducible contractions to
epinephrine and norepinephrine after equilibration in oxygenated Krebs
bicarbonate solution. I had at first planned to study the effects of
disturbances in energy-metabolism on these contractions, but I became much more
interested in using the aortic strip for studies on drug-receptor interactions.
By 1953, I had published a paper entitled "Reactions of strips of rabbit
aorta to epinephrine, isoproterenol, sodium nitrite and other drugs".
Among the other drugs was acetylcholine. I found that it only produced
contractions, whether it was added to resting strips or strips precontracted
with some other agent. That was a paradoxical response since acetylcholine was
known to be a very potent vasodilator in
vivo. Little did I suspect then what I was able to show many years later -
namely, that relaxation of arteries by acetylcholine is strictly
endothelium-dependent, and that my method of preparing the strips inadvertently
resulted in the mechanical removal of all the endothelial cells.
In 1954, I published a paper on the use of dibenamine in differentiating
receptors in the aortic strip, and in 1955 a review in Pharmacological Reviews
on the pharmacology of vascular smooth muscle. In that review, I tried to
develop receptor theory as a logical base for interpreting the responses of
vascular smooth muscle to many neuro transmitters, hormones and drugs. In order
to derive equations to account for the very slow onset and offset kinetics of
competitive antagonists as compared to the fast kinetics of agonists, I
developed a biophase model in which the agents moved between an aqueous
extracellular phase and a lipid membrane phase containing the receptors.
Although I paid homage in my review to A. J. Clark for his pioneering work in
developing receptor theory, I took issue with his hypothesis that response of a
tissue to an agonist is proportional to the fraction of receptors occupied by
the agonist. Our results with dibenamine, which behaved as an irreversible
competitive blocking agent of adrenergic ![]() -receptors,
had indicated that with a strong agonist like epinephrine, one could still
achieve well over half of the maximum contraction when only a small fraction of
receptors were still active. This was the beginning of my interest in the
concept of "receptor reserve" or "spare receptors." (A year
later, R.P. Stephenson published his classic paper on the subject in which he
introduced the concepts of efficacy, full agonist and partial agonist.)
-receptors,
had indicated that with a strong agonist like epinephrine, one could still
achieve well over half of the maximum contraction when only a small fraction of
receptors were still active. This was the beginning of my interest in the
concept of "receptor reserve" or "spare receptors." (A year
later, R.P. Stephenson published his classic paper on the subject in which he
introduced the concepts of efficacy, full agonist and partial agonist.)
In the review of 1955, I also briefly reported on a newly discovered phenomenon
- namely, that strips of rabbit aorta undergo reversible relaxation when
exposed to light of proper wavelength and intensity. This photorelaxation was
an accidental discovery that came from the observation that in one experiment
active contractile tone of two strips in one pair of organ chambers fluctuated
with time, whereas that of two strips in another pair of chambers remained
steady. The two strips showing fluctuations did so synchronously. Those two
strips, but not the other two, were in organ chambers near a window through
which they were exposed to skylight. Suspecting that the fluctuations in tone
were due to fluctuations in light intensity on the strips near the window (it
was a cloudy-bright day), I closed the shade on the window and both strips
increased in tone. I opened the shade and both decreased in tone. From that
point on, we never allowed our strips to be exposed to direct skylight. (The
usual overhead fluorescent lights do not produce photorelaxation.) Some studies
on the characteristics of photorelaxation were begun in St. Louis, and then
extended when I moved to Brooklyn.
In addition to working on in vitro
smooth muscle preparations at Washington University, I also began what became
many years of research on the pharmacology of an in vitro cardiac muscle preparation - namely the isolated
electrically-driven right atrium of the guinea pig. In starting that work, I
had the assistance of a very able technician, Taisija De Gubareff. Using
chemical and enzymatic methods for analysis of creatinine phosphate, ATP, ADP,
and AMP, we showed that neither development of "experimental failure"
in vitro (a steady loss of
contractile force over hours) nor recovery from failure on addition of a
cardiac glycoside was due to changes in concentration of these high-energy
phosphates. We also reported on the effects of anaerobiosis and of a number of
positive and negative inotropic agents. We collaborated with my good friend
William Sleator of the Physiology Department in the study of changes in
cellular action potentials (measured with intracellular microelectrodes)
associated with the changes in contractility of the guinea pig atrium in
response to epinephrine and acetylcholine, and a number of other inotropic
agents.
Suny Medical Center in Brooklyn (1956-)
In 1956, I accepted the position of chairman of the new Department of
Pharmacology at the State University of New York (SUNY) College of Medicine at
New York City (actually in Brooklyn, and later changed in name to SUNY
Downstate Medical Center and more recently to SUNY Health Science Center at
Brooklyn). The department had previously been part of a joint physiology and
pharmacology department headed by Chandler Brooks but with the opening of a
new, relatively huge (for the time) basic science building for the medical
school and with good financial support from the State University, there was
ample room and resources for a separate department. From the former joint
department, I inherited Julius Belford as an associate professor and Bernard
Mirkin as an assistant professor. For additional faculty, I recruited Kwang Soo
Lee, Leonard Procita, Lowell Greenbaum, Walter Wosilait and Arthur Zimmerman,
all in time for them to teach our first course for medical students. The
following year C. Y. Kao joined the staff. Also during the first year, we
accepted our first graduate students, namely Maurice Feinstein, who worked with
me, and Arnold Schwartz, who worked with Lee. During that year I didn't do much
bench work in the research lab since most of my time was spent organizing the
department and learning how to be a chairman. (I never became a well-organized
administrator and was always poor at delegating authority.)
In Brooklyn, I continued research on photorelaxation of blood vessels, factors
influencing contractility of cardiac muscle, peripheral adrenergic mechanisms,
and receptor theory and mechanisms. Then, about twenty-three years after moving
to Brooklyn, the research in my laboratory largely shifted to
endothelium-dependent relaxation of blood vessels. For convenience, I shall
divide the discussion of research in Brooklyn into subsections corresponding to
the areas that I have listed.
Photorelaxation of Blood Vessels
Helping with this research were Eugene Greenblatt, my first postdoctoral
fellow, and Stuart Ehrreich, my third graduate student. Among other things, we
were able to obtain an accurate action spectrum (with a peak at 310 nm) for the
photorelaxation. Later we observed that addition of sodium nitrite to the
bathing medium greatly sensitized the rabbit aortic strip to photorelaxation
and shifted the peak of the action spectrum to about 355 nm. Ehrreich and I
found that many other smooth muscle preparations (from stomach, intestine and
uterus) which did not ordinarily relax in response to radiation did so in the
presence of inorganic nitrite. Percy Lindgren, a visiting faculty member from
the Karolinska Institute, also worked with us for
a while on photosensitization by nitrite.
Many years later in the early 1980's, after the discovery of
endothelium-derived relaxing factor (EDRF), I again began research on
photorelaxation. Although photorelaxation did not depend on the presence of
endothelium on the strip or ring of rabbit aorta, we found many similarities
between it and endothelium-dependent relaxation (as produced by acetylcholine
or A23187). Not only was photorelaxation, like endothelium-dependent
relaxation, causally dependent on the elevation of cyclic GMP as a result of
stimulation of guanylate cyclase, but both were inhibited by hemoglobin and by
methylene blue. This work was carried out with Desingarao Jothianandan, who has
been a most helpful research associate in my lab over the past seventeen years.
Then, after EDRF was identified in 1986 as nitric oxide, Kazuki Matsunaga (a
postdoctoral fellow) and I reinvestigated the potentiation of photorelaxation
by sodium nitrite. Using a cleverly designed perfusion-bioassay type apparatus,
Matsunaga clearly demonstrated that the potentiation was due to the
photoactivated release of NO from nitrite. It is tempting to hypothesize that
light (in the absence of added nitrite) produces relaxation of vascular smooth
muscle by photoactivating the release of NO from some endogenous compound in
the muscle cell.
Factors Influencing Contractility of
Cardiac Muscle
My first graduate student in Brooklyn, Maurice Feinstein, did his Ph.D. thesis
research on the effects of experimental congestive heart failure, asphyxia and
ouabain on high energy phosphates and creatine content of the guinea pig heart.
My second graduate student, Albert Grossman, who began work in 1957, did his
thesis research on the effects of frequency of stimulation, extracellular
calcium concentration and various drugs on calcium exchange and contractility
of the guinea-pig left atrium. Grossman and I published three papers based on
his thesis research, which was one of the first attempts to determine the rates
of exchange of calcium (using 45Ca) between extracellular fluid and
various intracellular "pools" of calcium in cardiac muscle under
various conditions affecting contractility. We showed that the positive
inotropic effects of norepinephrine and strophanthin-K were correlated with an
increase in rate of exchange of calcium in an intracellular pool associated
with the contractile process and that the negative inotropic effects of
acetylcholine and adenosine were correlated with a decrease in rate of exchange
in that pool.
We also continued work with ryanodine, which produced a negative inotropic
effect on the guinea-pig atrium and actually changed the force-frequency effect
from a positive to negative staircase (mimicking the normal staircase in frog
heart). Sleator, De Gubareff and I had shown that the decrease in force with
ryanodine (unlike that with acetylcholine or adenosine) was not associated with
a decrease in duration of the action potential. The thesis research of Grossman
and a few years later that of another graduate student, Peter Wolf, also using 45Ca
to measure effects of ryanodine on calcium exchange, led to a hypothetical
model that fits fairly well with more recent work of others on the reactions of
ryanodine with "receptors" involved with calcium transport in the
sarcoplasmic reticulum.
Peripheral Adrenergic Mechanisms
In writing the 1955 review on the "Pharmacology of vascular smooth
muscle," I had become very interested in the mechanisms by which
sympathetic postganglionic denervation and certain drugs like cocaine markedly
potentiate the response of effector organs to epinephrine and norepinephrine,
yet markedly reduce the response to the sympathomimetic tyramine. My second
postdoctoral fellow, Sadashiv (Sada) Kirpekar, was assigned to work in this
area. He proved to be a gifted investigator, and we published a number of
papers together on work carried out between 1959 and 1962. In one paper, with
the running page heading of "the cocaine paradox," we presented
evidence that in aortic strips of rabbit and isolated electrically-driven atria
from guinea pig and cat, cocaine potentiated responses to norepinephrine and
inhibited those to tyramine by blocking one and the same site on adrenergic
nerve terminals. Blockade of this site inhibited the neuronal uptake of no
repinephrine from the region of the adrenergic receptors, thus potentiating its
action; however, blockade of the site also inhibited uptake of tyramine, whose
sympathomimetic action depends on release of norepinephrine from neuronal
storage sites, thus inhibiting its action. The site, which we called the
"transfer site" later became known as the uptake-1 (UI) site. In the
same paper we showed that reserpine, which depleted neuronal storage granules
of norepinephrine, did not interfere with activity of the uptake site. In
addition to Kirpekar, Peter Cervoni came in as a postdoctoral fellow to work on
peripheral adrenergic mechanisms. Both he and Kirpekar later became faculty
members in the department with Kirpekar staying on and becoming a stellar
figure in the field of adrenergic mechanisms before his untimely death in 1983.
In 1960, I was invited to present a paper on some of my studies on receptors
for sympathomimetic amines at a CIBA Foundation conference on Adrenergic
Mechanisms held at CIBA House in London. It was the occasion for my first trip
abroad and was very exciting. Among the many distinguished pharmacologists at
the conference were Sir Henry Dale, Sir John Gaddum and J.H. Burn. Burn at that
time was pushing his "cholinergic-link" hypothesis for norepinephrine
release at adrenergic nerve terminals. I felt strongly that he had misinterpreted
the experimental results which had led to the hypothesis and in the discussion
sessions I presented our own results with isolated atria which indicated that
there were nicotinic cholinergic receptors on adrenergic nerve terminals which
when stimulated by nicotine or acetylcholine triggered a transient release of
norepinephrine, but which played no role in release of norepinephrine on
electrical stimulation of the nerve.
In 1962-63, 1 spent a sabbatical year in the Department of Physiology of the
University of Geneva, where Jean Posternak was chairman. Although I did some
research and teaching there, I spent most of my time writing papers on research
that my colleagues and I had completed during the preceding few years and on a
review on receptor mechanisms (see below). I also visited a number of
laboratories in Europe where outstanding research on adrenergic mechanisms was
in progress. Among these were the laboratories of S. von
Euler in Stockholm, E. Muscholl in Mainz and John Gillespie in
Glasgow.
Between 1965 and 1970 I was fortunate in having a number of very competent
coworkers in research on peripheral adrenergic mechanisms. In addition to
Kirpekar, there were Pedro Sanchez-Garcia, (a visiting research associate who
later became a leading pharmacologist in his native Spain), Jerome Levin (a
postdoctoral fellow) and Arun Wakade (a graduate student who later became a
faculty member).
In early 1971, I began my second sabbatical leave, this time at the relatively
new medical school of the University of California at San Diego (located in La
Jolla). I became a visiting professor in Steve Mayer's Pharmacology Division of
the Department of Medicine. One reason for this choice of a sabbatical site was
that I wanted to learn the method for analysis of cyclic AMP that Mayer had
developed (this was before the development of radioimmunoassays for cyclic
nucleotides). However, I did not do a lot of research at La Jolla, partly because
a fair amount of my time that year was devoted to duties as president of the
American Society for Pharmacology and Experimental Therapeutics.
On returning from La Jolla to Brooklyn in 1972, I continued research on the
role of receptors located on prejunctional terminals (varicosities) of
adrenergic nerves. I collaborated with Kirpekar in an attempt to characterize
the inhibitory prejunctional ![]() -adrenergic
receptors on the nerve terminals in cat spleen. At the same time, one of my
graduate students, Odd Steinsland, was conducting a very exciting thesis
project on cholinergic receptors on prejunctional adrenergic nerve terminals in
the isolated, perfused central ear artery of the rabbit. He first
pharmacologically characterized with the use of various muscarinic agonists and
antagonists the prejunctional receptor through which acetylcholine produces a
marked inhibition of norepinephrine release (monitored by both the degree of
vasoconstriction and [3H]norepinephrine release). He then went on to
study the release of norepinephrine from the adrenergic neurons in the ear
artery by cholinergic agonists acting on prejunctional nicotinic receptors. At
the same time I was continuing studies, with the assistance of Taruna Wakade,
on the pharmacology of cholinergic nicotinic receptors on adrenergic
prejunctional terminals in the guinea-pig left atrium.
-adrenergic
receptors on the nerve terminals in cat spleen. At the same time, one of my
graduate students, Odd Steinsland, was conducting a very exciting thesis
project on cholinergic receptors on prejunctional adrenergic nerve terminals in
the isolated, perfused central ear artery of the rabbit. He first
pharmacologically characterized with the use of various muscarinic agonists and
antagonists the prejunctional receptor through which acetylcholine produces a
marked inhibition of norepinephrine release (monitored by both the degree of
vasoconstriction and [3H]norepinephrine release). He then went on to
study the release of norepinephrine from the adrenergic neurons in the ear
artery by cholinergic agonists acting on prejunctional nicotinic receptors. At
the same time I was continuing studies, with the assistance of Taruna Wakade,
on the pharmacology of cholinergic nicotinic receptors on adrenergic
prejunctional terminals in the guinea-pig left atrium.
Receptor Theory and Mechanisms
When I first gave a course on receptor theory and mechanisms to graduate
students in 1957-1958, the literature on the subject was relatively sparse:
papers by Clark, Gaddum, Schild, Arins, Stephenson, Nickerson and myself. I
became interested in developing suitable theory (occupation theory) and in vitro procedures for differentiating
and characterizing receptors. In particular, I concentrated on receptors for
adrenergic and cholinergic agents using as test tissues the rabbit aortic
strip, duodenal segment, and stomach fundus muscle, and the guinea-pig
electrically driven left atrium and tracheal ring.
In 1963, toward the end of my sabbatical year at the University of Geneva, I
completed a review on "Receptor Mechanisms" for Volume 4 of the
Annual Review of Pharmacology. In it, I took the opportunity to stress the
importance of Stephenson's ideas on efficacy and spare receptors. In 1965 at a
symposium on receptor mechanisms at Chelsea College in London, I presented a
paper on the use of ![]() -haloalkylamines,
as irreversible receptor antagonists, in the differentiation of receptors and
in the determination of dissociation constants of receptor-agonist complexes.
Using a slightly modified form of Stephenson's equations and introducing a
term,
-haloalkylamines,
as irreversible receptor antagonists, in the differentiation of receptors and
in the determination of dissociation constants of receptor-agonist complexes.
Using a slightly modified form of Stephenson's equations and introducing a
term, ![]() ,
for intrinsic efficacy, I derived a simple equation that predicted that the
slope and ordinate intercept of a double reciprocal plot of equiactive concentrations
of an agonist before and after irreversible inactivation of a fraction of its
receptors, could permit the determination of both the fraction of receptors
still active as well as the dissociation constant (KA) of the
agonist-receptor complex. For different agonists acting on the same receptor,
one could calculate from the KA values the fractional occupation by
each to obtain the same standard response before receptor inactivation, and
thus obtain relative efficacies. Using this approach, Paula (Bursztyn) Goldberg
(a graduate student) and I compared the dissociation constants and relative
efficacies of agonists acting on muscarinic cholinergic receptors of isolated
strips of rabbit stomach fundus muscle; and later John Besse (a postdoctoral
fellow) and I compared the dissociation constants and relative efficacies of
agonists acting on
,
for intrinsic efficacy, I derived a simple equation that predicted that the
slope and ordinate intercept of a double reciprocal plot of equiactive concentrations
of an agonist before and after irreversible inactivation of a fraction of its
receptors, could permit the determination of both the fraction of receptors
still active as well as the dissociation constant (KA) of the
agonist-receptor complex. For different agonists acting on the same receptor,
one could calculate from the KA values the fractional occupation by
each to obtain the same standard response before receptor inactivation, and
thus obtain relative efficacies. Using this approach, Paula (Bursztyn) Goldberg
(a graduate student) and I compared the dissociation constants and relative
efficacies of agonists acting on muscarinic cholinergic receptors of isolated
strips of rabbit stomach fundus muscle; and later John Besse (a postdoctoral
fellow) and I compared the dissociation constants and relative efficacies of
agonists acting on ![]() 1-adrenergic
receptors of rabbit aorta. In light of what is now known about receptor
signalling pathways through G-proteins, it is probably better to admit that the
pharmacological procedure which we developed for obtaining agonist-receptor
dissociation constants can only give approximate relative values. Nevertheless,
the procedure has proven useful in a number of studies.
1-adrenergic
receptors of rabbit aorta. In light of what is now known about receptor
signalling pathways through G-proteins, it is probably better to admit that the
pharmacological procedure which we developed for obtaining agonist-receptor
dissociation constants can only give approximate relative values. Nevertheless,
the procedure has proven useful in a number of studies.
In 1972, I published a review entitled "The classification of
adrenoceptors (adrenergic receptors). An evaluation from the standpoint of
receptor theory". In it I attempted to formulate the methods and necessary
conditions for the classification and differentiation of receptors by
pharmacological procedures designed to give accurate dissociation constants of
competitive antagonists, acting on a given receptor, and accurate relative
potencies and, if possible, dissociation constants of agonists acting on the
same receptor. In particular, I attempted to point out pitfalls in such
procedures and how to avoid them. For example, I derived theoretical equations
to illustrate how removal of the agonist from the region of the receptor by
active uptake or enzymatic destruction could markedly alter the slope of a
Schild plot for competitive antagonism from the theoretical slope of 1. Later,
Aaron Jurkiewicz, a visiting research associate from Sao Paulo, Niede
Jurkiewicz and I successfully used these theoretical equations in the analysis
of propranolol antagonism to isoproterenol in guinea-pig tracheal strips before
and after blockade of removal of the agonist by active uptake.
In 1977, I organized for the annual FASEB meeting a symposium on receptors. By
then binding of radioligands (usually 3H-labelled competitive
antagonists) had been used for several years for quantifying specific receptors
in membranes from homogenized cells and for determining the dissociation
constants of competitive antagonists and agonists for those receptors. Most of
the papers at the symposium were reports of studies with radioligands (e.g., R.
J. Lefkowitz on both ![]() -and
-and
![]() -adrenergic
receptors; P. Seeman on dopamine receptors; S. Snyder and colleagues on
serotonin receptors and opiate receptors). My paper at the symposium was partly
a discussion of how pharmacological procedures for differentiating and
characterizing receptors based on occupation theory were still very useful in
conjunction with the exciting new developments in receptor research being made
with specific radioligands.
-adrenergic
receptors; P. Seeman on dopamine receptors; S. Snyder and colleagues on
serotonin receptors and opiate receptors). My paper at the symposium was partly
a discussion of how pharmacological procedures for differentiating and
characterizing receptors based on occupation theory were still very useful in
conjunction with the exciting new developments in receptor research being made
with specific radioligands.
Also, I reviewed work that had been carried out in my laboratory on ![]() -adrenergic
receptors mediating relaxation of guinea-pig tracheal smooth muscle, and
presented results of pharmacological experiments that showed that this smooth
muscle did not have exclusively the
-adrenergic
receptors mediating relaxation of guinea-pig tracheal smooth muscle, and
presented results of pharmacological experiments that showed that this smooth
muscle did not have exclusively the ![]() 2-type of the
2-type of the ![]() -adrenergic
receptor, as dogma of that time would have it, but had an admixture of the
-adrenergic
receptor, as dogma of that time would have it, but had an admixture of the ![]() 1-type
as well - usually as a small fraction of the total of
1-type
as well - usually as a small fraction of the total of ![]() -receptors,
but, depending on the guinea-pig used, sometimes much more.
-receptors,
but, depending on the guinea-pig used, sometimes much more.
Endo Thelium-dependent Relaxation
Having obtained pharmacological evidence that guinea-pig tracheal smooth muscle
sometimes has a sizeable fraction of the ![]() 1-type
adrenergic receptor along with the
1-type
adrenergic receptor along with the ![]() 2-type
(see above), I decided that it would be well to reexamine the smooth muscle of
rabbit thoracic aorta to see if it also might have varying amounts of the
2-type
(see above), I decided that it would be well to reexamine the smooth muscle of
rabbit thoracic aorta to see if it also might have varying amounts of the ![]() 1-type
receptor mixed with the
1-type
receptor mixed with the ![]() 2-type. However, in the very first
experiment designed for this new study in May 1978, an accidental finding as a
result of a technician's error completely changed the course of research in my
laboratory. The accidental finding was that on the preparation of rabbit aorta
being used in the experiment, the muscarinic agents acetylcholine and carbachol
induced relaxation rather than the expected contraction. Why this accidental
finding was so exciting, how it led to our discovery of the endothelium-derived
relaxing factor (EDRF), and how that factor was eventually identified as nitric
oxide will not be discussed here since those matters will be considered in
detail in my Nobel Lecture.
2-type. However, in the very first
experiment designed for this new study in May 1978, an accidental finding as a
result of a technician's error completely changed the course of research in my
laboratory. The accidental finding was that on the preparation of rabbit aorta
being used in the experiment, the muscarinic agents acetylcholine and carbachol
induced relaxation rather than the expected contraction. Why this accidental
finding was so exciting, how it led to our discovery of the endothelium-derived
relaxing factor (EDRF), and how that factor was eventually identified as nitric
oxide will not be discussed here since those matters will be considered in
detail in my Nobel Lecture.
In 1982, I resigned from the chairmanship of the Department of Pharmacology at
the SUNY Downstate Medical Center, but continued as a professor. In 1989, I
retired from my professorship (receiving emeritus status), so that I now was
free of teaching duties and committee work related to the medical curriculum
but could still continue research in the department. My retirement also now
allowed me to spend about three and a half months each winter as an adjunct
Professor in the Department of Molecular and Cellular Pharmacology of the
University of Miami School of Medicine. Most of my time there I have spent
trying to catch up on the writing of manuscripts and on the reading of the
burgeoning literature in the field of nitric oxide research - an impossible
task these days! During the winter sojourns in Miami, I keep in touch with what
is going on in my research laboratory in Brooklyn by means of an occasional
visit, but mainly by frequent fax and telephone communications with my one or
two coworkers there. I consider myself very fortunate in having this
Brooklyn-Miami arrangement. Of course, an additional advantage for my wife
Maggie and me is that the arrangement allows us to enjoy the very pleasant
winter weather in Miami and some of the outdoor activities that it fosters
(golf, for instance, in my case).
From 1982 until the present writing, I have been the recipient of a number of
honors and awards for my research. Naturally, I have been very pleased to be
the recipient. Yet, in thinking back about what aspects of my research have
given me the greatest pleasure, I would not place the honors and awards first.
I think that my greatest pleasure has come from each first demonstration in my
laboratory that experiments designed to test a new hypothesis developed to
explain some earlier, often puzzling or paradoxical finding, have given results
consistent with the hypothesis. It is not just the immediate pleasure of
obtaining such results but also the anticipated pleasure of discussing the
results with others doing research in the same area - obviously an ego
supportive aspect.
I still enjoy doing bench work in the laboratory with my co-workers. The
research still is rather "old fashion" pharmacological research. I
was very lucky to stumble on unexpected results in 1978 that led to the finding
of endothelium-dependent relaxation and EDRF, and eventually to NO; for if I
had not, I would probably have still concentrated on research on receptor
theory and mechanisms, and been left far behind by others in that field who
have so brilliantly and successfully developed and used molecular biological
and other advanced methodologies in their research.
From Les Prix
Nobel. The Nobel Prizes 1998, Editor Tore Frngsmyr, [Nobel
Foundation], Stockholm, 1999
This
autobiography/biography was written at the time of the award and later
published in the book series Les Prix
Nobel/Nobel
Lectures. The information
is sometimes updated with an addendum submitted by the Laureate.
Robert F. Furchgott died on 19 May, 2009.
Walter Bortz II


 From
Wikipedia,
From
Wikipedia,
DR.
Walter Borst is a medical doctor, 85 years old. He believes in the serenity prayer. The prayer acknowledges that some things
are out of our control, like aging, but others like fitness are in our
control. We can control what
we put into our mouths and what comes out of our mouths. We can elect to be fit; all we have to
do is work for it. He is a professor at Stanford University
School of Medicine. He writes books
and runs a marathon every year with his wife.
https://www.youtube.com/watch?v=gtQBKfTjl5s
the free encyclopedia
Jump to: navigation, search
|
Walter Bortz |
|
|
Born |
20 March 1930 |
|
Nationality |
American |
|
Williams
College |
|
|
Known for |
Expertise on Aging and Diabetes |
Walter Michael Bortz II, (born March 20, 1930) is an American physician and author who teaches medicine at Stanford University. He is one of America's leading scientific experts on aging, and promotes the possibility of a 100-year lifespan.[1]
Contents |
Education
Dr. Bortz attended the Episcopal Academy of Philadelphia in 1947. He graduated from Williams College with a B.A., cum laude in 1951 and graduated from the University of Pennsylvania School of Medicine in 1955.
Career
Dr. Bortz is a former co-chairman of the American Medical AssociationÕs Task Force on Aging, past President of The American Geriatrics Society and is Chairman of the Medical Advisory Board for the Diabetes Research and Wellness Foundation.
His research focuses on the importance of physical exercise during the process of aging. Dr. Bortz has written 150 scientific articles for research publications such as JAMA, Annals of Internal Medicine, and Journal of Biological Chemistry, as well as articles in The New York Times, Washington Post, San Francisco Chronicle, The New England Journal of Medicine, American Journal of Public Health, and Town & Country.
He has written six books--We Live Too Short and Die Too Long, Dare to be 100, Living Longer for Dummies, Diabetes Danger, Next Medicine, and Occupy Medicine.
Personal life
Dr. Bortz is the son of Ed Bortz, former president of the American Medical Association.[2] Dr. Bortz lives with his wife in Portola Valley, California. He has four children and nine grandchildren.
A runner for several decades, Bortz runs over 10 miles per week and has finished in over 30 marathons, including the New York and Boston marathons.
Awards
- Paavo Nurmi Award, Runners World Magazine 1986
- University of California, San Francisco - Institute for Health & Aging, Distinguished Leadership Award 1990
- Kenneth Cooper Award for Scientific Contribution to Active Living George Sheehan Award - National Fitness Leader's Association 1996
- American Society on Aging, Presidential Award 2002
- Avenidas Lifetime Achievement Award, Palo Alto, CA 2007
References
1. ^ Reviewed in Levin-Scherz, J. (March 23/30, 2011) Journal of the American Medical Association 305(12):1244 and Krall, R.L. (April 8, 2011) "A Call to Reorient Healthcare" Science 332:177
2. ^ "Walter Bortz Podcast". Stanford University. Retrieved 2013-01-29.